Spectral and temporal modulation tradeoff in the inferior colliculus
- PMID: 20018831
- PMCID: PMC2822687
- DOI: 10.1152/jn.00813.2009
Spectral and temporal modulation tradeoff in the inferior colliculus
Abstract
The cochlea encodes sounds through frequency-selective channels that exhibit low-pass modulation sensitivity. Unlike the cochlea, neurons in the auditory midbrain are tuned for spectral and temporal modulations found in natural sounds, yet the role of this transformation is not known. We report a distinct tradeoff in modulation sensitivity and tuning that is topographically ordered within the central nucleus of the inferior colliculus (CNIC). Spectrotemporal receptive fields (STRFs) were obtained with 16-channel electrodes inserted orthogonal to the isofrequency lamina. Surprisingly, temporal and spectral characteristics exhibited an opposing relationship along the tonotopic axis. For low best frequencies (BFs), units were selective for fast temporal and broad spectral modulations. A systematic progression was observed toward slower temporal and finer spectral modulation sensitivity at high BF. This tradeoff was strongly reflected in the arrangement of excitation and inhibition and, consequently, in the modulation tuning characteristics. Comparisons with auditory nerve fibers show that these trends oppose the pattern imposed by the peripheral filters. These results suggest that spectrotemporal preferences are reordered within the tonotopic axis of the CNIC. This topographic organization has profound implications for the coding of spectrotemporal features in natural sounds and could underlie a number of perceptual phenomena.
Figures

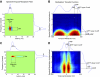
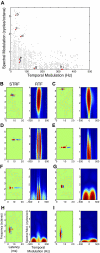

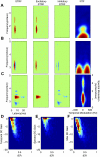
 , single units). Low-frequency units were concentrated about low spectral resolution (low bSMF) and fast temporal modulations (high bTMF). Accordingly, the population RTFs for low frequencies consisted of a dominant response lobe that extended to high bTMF values and had little energy for bSMF >0.5 cycle/octave (e.g., A and B). In contrast high-frequency sites (e.g., F–H) prefer finer spectral (high bSMF) and slower temporal modulations (low bTMF). This was evident both in the density of units as well as the energy distribution for the population RTF for each frequency band. A smooth continuum was observed such that spectrotemporal preferences varied systematically with increasing BF.
, single units). Low-frequency units were concentrated about low spectral resolution (low bSMF) and fast temporal modulations (high bTMF). Accordingly, the population RTFs for low frequencies consisted of a dominant response lobe that extended to high bTMF values and had little energy for bSMF >0.5 cycle/octave (e.g., A and B). In contrast high-frequency sites (e.g., F–H) prefer finer spectral (high bSMF) and slower temporal modulations (low bTMF). This was evident both in the density of units as well as the energy distribution for the population RTF for each frequency band. A smooth continuum was observed such that spectrotemporal preferences varied systematically with increasing BF.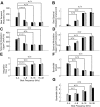
 , median values for single units; ■, multi units. A and C: median best temporal modulation and upper cutoffs decrease, while median best spectral modulation and upper cutoffs increase with increasing BF (B and D). A related trend is also observed for the STRF integration time and proportional bandwidth (in octaves). Median integration times (E) and proportional bandwidths (F) exhibit an opposing trend with increasing BF. Temporal integration times are shorter and spectral bandwidths (in octaves) are broader for low-frequency sites (2–4 kHz). G: in contrast, absolute bandwidths (Hz) increase with increasing BF. *, designate significant comparisons (Wilcoxon rank sum, P < 0.05).
, median values for single units; ■, multi units. A and C: median best temporal modulation and upper cutoffs decrease, while median best spectral modulation and upper cutoffs increase with increasing BF (B and D). A related trend is also observed for the STRF integration time and proportional bandwidth (in octaves). Median integration times (E) and proportional bandwidths (F) exhibit an opposing trend with increasing BF. Temporal integration times are shorter and spectral bandwidths (in octaves) are broader for low-frequency sites (2–4 kHz). G: in contrast, absolute bandwidths (Hz) increase with increasing BF. *, designate significant comparisons (Wilcoxon rank sum, P < 0.05).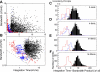


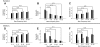
 , median values to single units; ▪, multiunits. The excitatory (A) and inhibitory (D) integration time both increase with BF while STRF excitatory bandwidths (in octaves) decrease (B). In contrast, absolute excitatory bandwidths increase with BF (C). The corresponding bandwidth trends for the inhibitory STRF component (E and F) were less systematic and differed somewhat between single and multiunits. *, significant comparisons (Wilcoxon rank sum, P < 0.05).
, median values to single units; ▪, multiunits. The excitatory (A) and inhibitory (D) integration time both increase with BF while STRF excitatory bandwidths (in octaves) decrease (B). In contrast, absolute excitatory bandwidths increase with BF (C). The corresponding bandwidth trends for the inhibitory STRF component (E and F) were less systematic and differed somewhat between single and multiunits. *, significant comparisons (Wilcoxon rank sum, P < 0.05).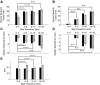
 , single units; ▪, multiunits. Median temporal (C) and spectral (D) DC gains decrease with BF (Wilcoxon rank sum, P < 0.05) consistent with stronger temporal and spectral band-pass modulation tuning for higher frequency sites. The inhibitory-to-excitatory power ratio (IER) does not exhibit a significant increase for higher frequency sites (Wilcoxon rank sum, N.S.). *, significant comparisons (P < 0.05).
, single units; ▪, multiunits. Median temporal (C) and spectral (D) DC gains decrease with BF (Wilcoxon rank sum, P < 0.05) consistent with stronger temporal and spectral band-pass modulation tuning for higher frequency sites. The inhibitory-to-excitatory power ratio (IER) does not exhibit a significant increase for higher frequency sites (Wilcoxon rank sum, N.S.). *, significant comparisons (P < 0.05).References
-
- Adams JC. Ascending projections to the inferior colliculus. J Comp Neurol 183: 519–538, 1979 - PubMed
-
- Attias H, Schreiner C. Low-order temporal statistics of natural sounds. Adv Neural Inform Process Syst 9: 27–33, 1998a
-
- Attias H, Schreiner C. Coding of naturalistic stimuli by auditory midbrain neurons. Adv Neural Inform Process Syst 10: 103–109, 1998b
-
- Batra R, Fitzpatrick DC. Discharge patterns of neurons in the ventral nucleus of the lateral lemniscus of the unanesthetized rabbit. J Neurophysiol 82: 1097–1113, 1999 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

