Identification of the minimal functional unit of the homo-oligomeric human reduced folate carrier
- PMID: 20018840
- PMCID: PMC2836078
- DOI: 10.1074/jbc.M109.086033
Identification of the minimal functional unit of the homo-oligomeric human reduced folate carrier
Abstract
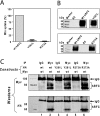
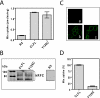
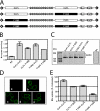
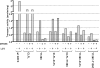
The reduced folate carrier (RFC) is the major transport system for folates in mammals. We previously demonstrated the existence of human RFC (hRFC) homo-oligomers and established the importance of these higher order structures to intracellular trafficking and carrier function. In this report, we examined the operational significance of hRFC oligomerization and the minimal functional unit for transport. In negative dominance experiments, multimeric transporters composed of different ratios of active (either wild type (WT) or cysteine-less (CLFL)) and inactive (either inherently inactive (Y281L and R373A) due to mutation, or resulting from inactivation of the Y126C mutant by (2-sulfonatoethyl) methanethiosulfonate (MTSES)) hRFC monomers were expressed in hRFC-null HeLa (R5) cells, and residual WT or CLFL activity was measured. In either case, residual transport activity with increasing levels of inactive mutant correlated linearly with the fraction of WT or CLFL hRFC in plasma membranes. When active covalent hRFC dimers, generated by fusing CLFL and Y126C monomers, were expressed in R5 cells and treated with MTSES, transport activity of the CLFL-CLFL dimer was unaffected, whereas Y126C-Y126C was potently (64%) inhibited; heterodimeric CLFL-Y126C and Y126C-CLFL were only partly (27 and 23%, respectively) inhibited by MTSES. In contrast to Y126C-Y126C, trans-stimulation of methotrexate uptake by intracellular folates for Y126C-CLFL and CLFL-Y126C was nominally affected by MTSES. Collectively, these results strongly support the notion that each hRFC monomer comprises a single translocation pathway for anionic folate substrates and functions independently of other monomers (i.e. despite an oligomeric structure, hRFC functions as a monomer).
Figures
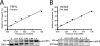
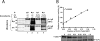
Similar articles
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
-
Oligomeric structure of the human reduced folate carrier: identification of homo-oligomers and dominant-negative effects on carrier expression and function.J Biol Chem. 2009 Jan 30;284(5):3285-3293. doi: 10.1074/jbc.M807206200. Epub 2008 Nov 19. J Biol Chem. 2009. PMID: 19019821 Free PMC article.
-
Localization of a substrate binding domain of the human reduced folate carrier to transmembrane domain 11 by radioaffinity labeling and cysteine-substituted accessibility methods.J Biol Chem. 2005 Oct 28;280(43):36206-13. doi: 10.1074/jbc.M507295200. Epub 2005 Aug 22. J Biol Chem. 2005. PMID: 16115875
-
Transmembrane domains 4, 5, 7, 8, and 10 of the human reduced folate carrier are important structural or functional components of the transmembrane channel for folate substrates.J Biol Chem. 2006 Nov 3;281(44):33588-96. doi: 10.1074/jbc.M607049200. Epub 2006 Aug 21. J Biol Chem. 2006. PMID: 16923800
-
Human reduced folate carrier: translation of basic biology to cancer etiology and therapy.Cancer Metastasis Rev. 2007 Mar;26(1):111-28. doi: 10.1007/s10555-007-9046-2. Cancer Metastasis Rev. 2007. PMID: 17334909 Review.
Cited by
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
-
Transmembrane topology and oligomeric structure of the high-affinity choline transporter.J Biol Chem. 2012 Dec 14;287(51):42826-34. doi: 10.1074/jbc.M112.405027. Epub 2012 Nov 6. J Biol Chem. 2012. PMID: 23132865 Free PMC article.
-
Differential roles of cysteine residues in the cellular trafficking, dimerization, and function of the high-density lipoprotein receptor, SR-BI.Biochemistry. 2011 Dec 20;50(50):10860-75. doi: 10.1021/bi201264y. Epub 2011 Nov 29. Biochemistry. 2011. PMID: 22097902 Free PMC article.
-
The human proton-coupled folate transporter: Biology and therapeutic applications to cancer.Cancer Biol Ther. 2012 Dec;13(14):1355-73. doi: 10.4161/cbt.22020. Epub 2012 Sep 6. Cancer Biol Ther. 2012. PMID: 22954694 Free PMC article. Review.
-
Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter.J Biol Chem. 2012 Feb 10;287(7):4982-95. doi: 10.1074/jbc.M111.306860. Epub 2011 Dec 16. J Biol Chem. 2012. PMID: 22179615 Free PMC article.
References
-
- Stokstad E. L. R. (1990) in Folic Acid Metabolism in Health and Disease (Picciano M. F., Stokstad E. L. R., Greogory J. F. ed) pp. 1–21, Wiley-Liss, New York
-
- Sirotnak F. M., Tolner B. (1999) Annu. Rev. Nutr. 19, 91–122 - PubMed
-
- Matherly L. H., Goldman D. I. (2003) Vitam. Horm. 66, 403–456 - PubMed
-
- Matherly L. H., Hou Z., Deng Y. (2007) Cancer Metastasis Rev. 26, 111–128 - PubMed
-
- Goldman I. D. (1971) Ann. N.Y. Acad. Sci. 186, 400–422 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

