Growth factor regulation of prostaglandin-endoperoxide synthase 2 (Ptgs2) expression in colonic mesenchymal stem cells
- PMID: 20018844
- PMCID: PMC2836106
- DOI: 10.1074/jbc.M109.032672
Growth factor regulation of prostaglandin-endoperoxide synthase 2 (Ptgs2) expression in colonic mesenchymal stem cells
Abstract
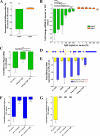
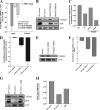
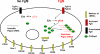
We previously found that a population of colonic stromal cells that constitutively express high levels of prostaglandin-endoperoxide synthase 2 (Ptgs2, also known as Cox-2) altered their location in the lamina propria in response to injury in a Myd88-dependent manner (Brown, S. L., Riehl, T. E., Walker, M. R., Geske, M. J., Doherty, J. M., Stenson, W. F., and Stappenbeck, T. S. (2007) J. Clin. Invest. 117, 258-269). At the time of this study, the identity of these cells and the mechanism by which they expressed high levels of Ptgs2 were unknown. Here we found that these colonic stromal cells were mesenchymal stem cells (MSCs). These colonic MSCs expressed high Ptgs2 levels not through interaction with bacterial products but instead as a consequence of mRNA stabilization downstream of Fgf9 (fibroblast growth factor 9), a growth factor that is constitutively expressed by the intestinal epithelium. This stabilization was mediated partially through a mechanism involving endogenous CUG-binding protein 2 (CUGbp2). These studies suggest that Fgf9 is an important factor in the regulation of Ptgs2 in colonic MSCs and may be a factor involved in its constitutive expression in vivo.
Figures

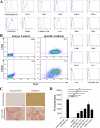
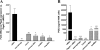
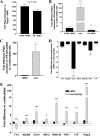
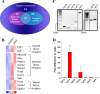
Similar articles
-
Igf2bp1 is required for full induction of Ptgs2 mRNA in colonic mesenchymal stem cells in mice.Gastroenterology. 2012 Jul;143(1):110-21.e10. doi: 10.1053/j.gastro.2012.03.037. Epub 2012 Mar 27. Gastroenterology. 2012. PMID: 22465430 Free PMC article.
-
Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury.J Clin Invest. 2007 Jan;117(1):258-69. doi: 10.1172/JCI29159. J Clin Invest. 2007. PMID: 17200722 Free PMC article.
-
Novel intestinal splice variants of RNA-binding protein CUGBP2: isoform-specific effects on mitotic catastrophe.Am J Physiol Gastrointest Liver Physiol. 2008 Apr;294(4):G971-81. doi: 10.1152/ajpgi.00540.2007. Epub 2008 Feb 7. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18258790 Free PMC article.
-
Prostaglandin-endoperoxide synthase (PTGS1 and PTGS2) expression and prostaglandin production by normal monkey ovarian surface epithelium.Fertil Steril. 2006 Oct;86(4 Suppl):1088-96. doi: 10.1016/j.fertnstert.2006.03.022. Epub 2006 Sep 7. Fertil Steril. 2006. PMID: 16962117
-
CUGBP2 plays a critical role in apoptosis of breast cancer cells in response to genotoxic injury.Ann N Y Acad Sci. 2003 Dec;1010:504-9. doi: 10.1196/annals.1299.093. Ann N Y Acad Sci. 2003. PMID: 15033780
Cited by
-
Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner.Gut. 2012 Jun;61(6):829-38. doi: 10.1136/gutjnl-2011-300367. Epub 2011 Oct 24. Gut. 2012. PMID: 22027478 Free PMC article.
-
Colonic epithelial response to injury requires Myd88 signaling in myeloid cells.Mucosal Immunol. 2012 Mar;5(2):194-206. doi: 10.1038/mi.2011.65. Epub 2012 Jan 18. Mucosal Immunol. 2012. PMID: 22258450 Free PMC article.
-
Definitive Endodermal Cells Supply an in vitro Source of Mesenchymal Stem/Stromal Cells.Commun Biol. 2023 May 1;6(1):476. doi: 10.1038/s42003-023-04810-5. Commun Biol. 2023. PMID: 37127734 Free PMC article.
-
CELFish ways to modulate mRNA decay.Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):695-707. doi: 10.1016/j.bbagrm.2013.01.001. Epub 2013 Jan 15. Biochim Biophys Acta. 2013. PMID: 23328451 Free PMC article. Review.
-
Nonmicrobial Activation of TLRs Controls Intestinal Growth, Wound Repair, and Radioprotection.Front Immunol. 2021 Jan 21;11:617510. doi: 10.3389/fimmu.2020.617510. eCollection 2020. Front Immunol. 2021. PMID: 33552081 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

