A novel type of E3 ligase for the Ufm1 conjugation system
- PMID: 20018847
- PMCID: PMC2820770
- DOI: 10.1074/jbc.M109.036814
A novel type of E3 ligase for the Ufm1 conjugation system
Abstract
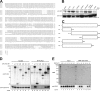
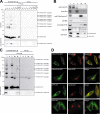
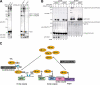
The ubiquitin fold modifier 1 (Ufm1) is the most recently discovered ubiquitin-like modifier whose conjugation (ufmylation) system is conserved in multicellular organisms. Ufm1 is known to covalently attach with cellular protein(s) via a specific E1-activating enzyme (Uba5) and an E2-conjugating enzyme (Ufc1), but its E3-ligating enzyme(s) as well as the target protein(s) remain unknown. Herein, we report both a novel E3 ligase for Ufm1, designated Ufl1, and an Ufm1-specific substrate ligated by Ufl1, C20orf116. Ufm1 was covalently conjugated with C20orf116. Although Ufl1 has no obvious sequence homology to any other known E3s for ubiquitin and ubiquitin-like modifiers, the C20orf116 x Ufm1 formation was greatly accelerated by Ufl1. The C20orf116 x Ufm1 conjugate was cleaved by Ufm1-specific proteases, implying the reversibility of ufmylation. The conjugation was abundant in the liver and lungs of Ufm1-transgenic mice, fractionated into membrane fraction, and impaired in Uba5 knock-out cells. Intriguingly, immunological analysis revealed localizations of Ufl1 and C20orf116 mainly to the endoplasmic reticulum. Our results provide novel insights into the Ufm1 system involved in cellular regulation of multicellular organisms.
Figures



References
-
- Hershko A., Ciechanover A. (1998) Annu. Rev. Biochem. 67, 425–479 - PubMed
-
- Mukhopadhyay D., Riezman H. (2007) Science 315, 201–205 - PubMed
-
- Pickart C. M., Eddins M. J. (2004) Biochim. Biophys. Acta 1695, 55–72 - PubMed
-
- Groettrup M., Pelzer C., Schmidtke G., Hofmann K. (2008) Trends Biochem. Sci. 33, 230–237 - PubMed
-
- Jin J., Li X., Gygi S. P., Harper J. W. (2007) Nature 447, 1135–1138 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

