JNK pathway-associated phosphatase dephosphorylates focal adhesion kinase and suppresses cell migration
- PMID: 20018849
- PMCID: PMC2820775
- DOI: 10.1074/jbc.M109.060186
JNK pathway-associated phosphatase dephosphorylates focal adhesion kinase and suppresses cell migration
Abstract
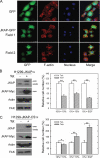
JNK pathway-associated phosphatase (JKAP, also named DUSP22) is expressed in various tissues, indicating that JKAP may have an important biological function. We showed that JKAP localized in the actin filament-enriched region. Expression of JKAP reduced cell migration, whereas a JKAP mutant lacking catalytic activity promoted cell motility. JKAP efficiently removed tyrosine phosphorylation of several proteins. We have identified focal adhesion kinase (FAK) as a substrate of JKAP. Overexpression of JKAP, but not JKAP mutant lacking catalytic activity, decreased FAK phosphorylation at tyrosines 397, 576, and 577 in H1299 cells. Consistent with these results, decreasing JKAP expression by RNA interference promoted cell migration and Src-induced FAK phosphorylation. Taken together, this study identified a new role for JKAP in the modulation of FAK phosphorylation and cell motility.
Figures




References
-
- Patterson K. I., Brummer T., O'Brien P. M., Daly R. J. (2009) Biochem. J. 418, 475–489 - PubMed
-
- Barford D., Flint A. J., Tonks N. K. (1994) Science 263, 1397–1404 - PubMed
-
- Neel B. G., Tonks N. K. (1997) Curr. Opin. Cell Biol. 9, 193–204 - PubMed
-
- Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. (2004) Cell 117, 699–711 - PubMed
-
- Chen A. J., Zhou G., Juan T., Colicos S. M., Cannon J. P., Cabriera-Hansen M., Meyer C. F., Jurecic R., Copeland N. G., Gilbert D. J., Jenkins N. A., Fletcher F., Tan T. H., Belmont J. W. (2002) J. Biol. Chem. 277, 36592–36601 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

