Human alanine-glyoxylate aminotransferase 2 lowers asymmetric dimethylarginine and protects from inhibition of nitric oxide production
- PMID: 20018850
- PMCID: PMC2820767
- DOI: 10.1074/jbc.M109.091280
Human alanine-glyoxylate aminotransferase 2 lowers asymmetric dimethylarginine and protects from inhibition of nitric oxide production
Abstract
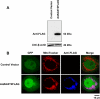
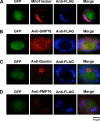
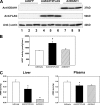
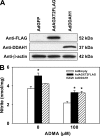
Elevated blood concentrations of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric-oxide (NO) synthase, are found in association with diabetes, hypertension, congestive heart failure, and atherosclerosis. ADMA levels are controlled by dimethylarginine dimethylaminohydrolases (DDAHs), cytosolic enzymes that hydrolyze ADMA to citrulline and dimethylamine. ADMA also has been proposed to be regulated through an alternative pathway by alanine-glyoxylate aminotransferase 2 (AGXT2), a mitochondrial aminotransferase expressed primarily in the kidney. The goal of this study was to define the subcellular localization of human AGXT2 and test the hypothesis that overexpression of human AGXT2 protects from ADMA-induced inhibition in nitric oxide (NO) production. AGXT2 was cloned from human kidney cDNA and overexpressed in COS-7 cells and human umbilical vein endothelial cells with a C-terminal FLAG epitope tag. Mitochondrial localization of human AGXT2 was demonstrated by confocal microscopy and a 41-amino acid N-terminal mitochondrial cleavage sequence was delineated by N-terminal sequencing of the mature protein. Overexpression of human AGXT2 in the liver of C57BL/6 mice using an adenoviral expression vector produced significant decreases in ADMA levels in plasma and liver. Overexpression of human AGXT2 also protected endothelial cells from ADMA-mediated inhibition of NO production. We conclude that mitochondrially localized human AGXT2 is able to effectively metabolize ADMA in vivo resulting in decreased ADMA levels and improved endothelial NO production.
Figures
Similar articles
-
Overexpression of alanine-glyoxylate aminotransferase 2 protects from asymmetric dimethylarginine-induced endothelial dysfunction and aortic remodeling.Sci Rep. 2022 Jun 7;12(1):9381. doi: 10.1038/s41598-022-13169-2. Sci Rep. 2022. PMID: 35672381 Free PMC article.
-
AGXT2 and DDAH-1 genetic variants are highly correlated with serum ADMA and SDMA levels and with incidence of coronary artery disease in Egyptians.Mol Biol Rep. 2018 Dec;45(6):2411-2419. doi: 10.1007/s11033-018-4407-1. Epub 2018 Oct 3. Mol Biol Rep. 2018. PMID: 30284143
-
Role of alanine:glyoxylate aminotransferase 2 in metabolism of asymmetric dimethylarginine in the settings of asymmetric dimethylarginine overload and bilateral nephrectomy.Nephrol Dial Transplant. 2014 Nov;29(11):2035-42. doi: 10.1093/ndt/gfu236. Epub 2014 Jul 6. Nephrol Dial Transplant. 2014. PMID: 25002409
-
Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets?Pharmacol Ther. 2013 Dec;140(3):239-57. doi: 10.1016/j.pharmthera.2013.07.004. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859953 Review.
-
AGXT2: An unnegligible aminotransferase in cardiovascular and urinary systems.J Mol Cell Cardiol. 2017 Dec;113:33-38. doi: 10.1016/j.yjmcc.2017.09.010. Epub 2017 Sep 29. J Mol Cell Cardiol. 2017. PMID: 28970090 Review.
Cited by
-
Metabolic Signatures in Coronary Artery Disease: Results from the BioHEART-CT Study.Cells. 2021 Apr 22;10(5):980. doi: 10.3390/cells10050980. Cells. 2021. PMID: 33922315 Free PMC article.
-
Circulating amino acids and amino acid-related metabolites and risk of breast cancer among predominantly premenopausal women.NPJ Breast Cancer. 2021 May 18;7(1):54. doi: 10.1038/s41523-021-00262-4. NPJ Breast Cancer. 2021. PMID: 34006878 Free PMC article.
-
Asymmetric Dimethylarginine Is a Well Established Mediating Risk Factor for Cardiovascular Morbidity and Mortality-Should Patients with Elevated Levels Be Supplemented with Citrulline?Healthcare (Basel). 2016 Jul 8;4(3):40. doi: 10.3390/healthcare4030040. Healthcare (Basel). 2016. PMID: 27417628 Free PMC article. Review.
-
Genome-wide association study of L-arginine and dimethylarginines reveals novel metabolic pathway for symmetric dimethylarginine.Circ Cardiovasc Genet. 2014 Dec;7(6):864-72. doi: 10.1161/CIRCGENETICS.113.000264. Epub 2014 Sep 21. Circ Cardiovasc Genet. 2014. PMID: 25245031 Free PMC article.
-
Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach.Int J Mol Sci. 2019 Jul 26;20(15):3668. doi: 10.3390/ijms20153668. Int J Mol Sci. 2019. PMID: 31357472 Free PMC article. Review.
References
-
- Vallance P., Leiper J. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1023–1030 - PubMed
-
- Böger R. H., Cooke J. P., Vallance P. (2005) Vasc. Med. 10, Suppl. 1, S1–S2 - PubMed
-
- Leiper J., Nandi M., Torondel B., Murray-Rust J., Malaki M., O'Hara B., Rossiter S., Anthony S., Madhani M., Selwood D., Smith C., Wojciak-Stothard B., Rudiger A., Stidwill R., McDonald N. Q., Vallance P. (2007) Nat. Med. 13, 198–203 - PubMed
-
- Wang D., Gill P. S., Chabrashvili T., Onozato M. L., Raggio J., Mendonca M., Dennehy K., Li M., Modlinger P., Leiper J., Vallance P., Adler O., Leone A., Tojo A., Welch W. J., Wilcox C. S. (2007) Circ. Res. 101, 627–635 - PubMed
-
- Kielstein J. T., Impraim B., Simmel S., Bode-Böger S. M., Tsikas D., Frölich J. C., Hoeper M. M., Haller H., Fliser D. (2004) Circulation 109, 172–177 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

