Myosin light chain kinase is necessary for tonic airway smooth muscle contraction
- PMID: 20018858
- PMCID: PMC2820780
- DOI: 10.1074/jbc.M109.062836
Myosin light chain kinase is necessary for tonic airway smooth muscle contraction
Abstract
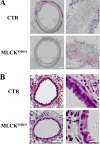
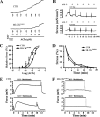
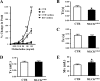
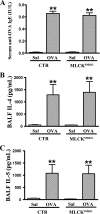
Different interacting signaling modules involving Ca(2+)/calmodulin-dependent myosin light chain kinase, Ca(2+)-independent regulatory light chain phosphorylation, myosin phosphatase inhibition, and actin filament-based proteins are proposed as specific cellular mechanisms involved in the regulation of smooth muscle contraction. However, the relative importance of specific modules is not well defined. By using tamoxifen-activated and smooth muscle-specific knock-out of myosin light chain kinase in mice, we analyzed its role in tonic airway smooth muscle contraction. Knock-out of the kinase in both tracheal and bronchial smooth muscle significantly reduced contraction and myosin phosphorylation responses to K(+)-depolarization and acetylcholine. Kinase-deficient mice lacked bronchial constrictions in normal and asthmatic airways, whereas the asthmatic inflammation response was not affected. These results indicate that myosin light chain kinase acts as a central participant in the contractile signaling module of tonic smooth muscle. Importantly, contractile airway smooth muscles are necessary for physiological and asthmatic airway resistance.
Figures




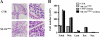
Similar articles
-
AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase.J Biol Chem. 2008 Jul 4;283(27):18505-12. doi: 10.1074/jbc.M802053200. Epub 2008 Apr 21. J Biol Chem. 2008. PMID: 18426792
-
Signaling through myosin light chain kinase in smooth muscles.J Biol Chem. 2013 Mar 15;288(11):7596-7605. doi: 10.1074/jbc.M112.427112. Epub 2013 Jan 28. J Biol Chem. 2013. PMID: 23362260 Free PMC article.
-
Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo.Am J Physiol Cell Physiol. 2008 Aug;295(2):C358-64. doi: 10.1152/ajpcell.90645.2007. Epub 2008 Jun 4. Am J Physiol Cell Physiol. 2008. PMID: 18524939 Free PMC article.
-
Myosin light chain kinase phosphorylation: regulation of the Ca2+ sensitivity of contractile elements.Adv Exp Med Biol. 1991;304:129-38. doi: 10.1007/978-1-4684-6003-2_12. Adv Exp Med Biol. 1991. PMID: 1803895 Review.
-
Phosphorylation of myosin light chain kinase: a cellular mechanism for Ca2+ desensitization.Mol Cell Biochem. 1993 Nov;127-128:229-37. doi: 10.1007/BF01076774. Mol Cell Biochem. 1993. PMID: 7935354 Review.
Cited by
-
Myosin light chain kinase (MLCK) regulates cell migration in a myosin regulatory light chain phosphorylation-independent mechanism.J Biol Chem. 2014 Oct 10;289(41):28478-88. doi: 10.1074/jbc.M114.567446. Epub 2014 Aug 13. J Biol Chem. 2014. PMID: 25122766 Free PMC article.
-
Diffusion of myosin light chain kinase on actin: A mechanism to enhance myosin phosphorylation rates in smooth muscle.J Gen Physiol. 2015 Oct;146(4):267-80. doi: 10.1085/jgp.201511483. J Gen Physiol. 2015. PMID: 26415568 Free PMC article.
-
Visualization of stimulus-specific heterogeneous activation of individual vascular smooth muscle cells in aortic tissues.J Cell Physiol. 2018 Jan;233(1):434-446. doi: 10.1002/jcp.25903. Epub 2017 Jul 14. J Cell Physiol. 2018. PMID: 28295256 Free PMC article.
-
Physiological signalling to myosin phosphatase targeting subunit-1 phosphorylation in ileal smooth muscle.J Physiol. 2016 Jun 15;594(12):3209-25. doi: 10.1113/JP271703. Epub 2016 Mar 4. J Physiol. 2016. PMID: 26847850 Free PMC article.
-
Biochemistry of smooth muscle myosin light chain kinase.Arch Biochem Biophys. 2011 Jun 15;510(2):135-46. doi: 10.1016/j.abb.2011.04.018. Epub 2011 May 3. Arch Biochem Biophys. 2011. PMID: 21565153 Free PMC article. Review.
References
-
- Golenhofen K., Mandrek K. (1991) Dig. Dis. 9, 341–346 - PubMed
-
- Somlyo A. V., Somlyo A. P. (1968) J. Pharmacol. Exp. Ther. 159, 129–145 - PubMed
-
- Ito M., Nakano T., Erdodi F., Hartshorne D. J. (2004) Mol. Cell. Biochem. 259, 197–209 - PubMed
-
- Kamm K. E., Stull J. T. (2001) J. Biol. Chem. 276, 4527–4530 - PubMed
-
- Murthy K. S. (2006) Annu. Rev. Physiol. 68, 345–374 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

