NF-kappaB functions in stromal fibroblasts to regulate early postnatal muscle development
- PMID: 20018862
- PMCID: PMC2820776
- DOI: 10.1074/jbc.M109.075606
NF-kappaB functions in stromal fibroblasts to regulate early postnatal muscle development
Abstract
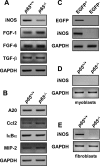
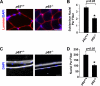
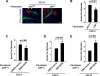
Classical NF-kappaB activity functions as an inhibitor of the skeletal muscle myogenic program. Recent findings reveal that even in newborn RelA/p65(-/-) mice, myofiber numbers are increased over that of wild type mice, suggesting that NF-kappaB may be a contributing factor in early postnatal skeletal muscle development. Here we show that in addition to p65 deficiency, repression of NF-kappaB with the IkappaB alpha-SR transdominant inhibitor or with muscle-specific deletion of IKKbeta resulted in similar increases in total fiber numbers as well as an up-regulation of myogenic gene products. Upon further characterization of early postnatal muscle, we observed that NF-kappaB activity progressively declines within the first few weeks of development. At birth, the majority of this activity is compartmentalized to muscle fibers, but by neonatal day 8 NF-kappaB activity from the myofibers diminishes, and instead, stromal fibroblasts become the main cellular compartment within the muscle that contains active NF-kappaB. We find that NF-kappaB functions in these fibroblasts to regulate inducible nitric-oxide synthase expression, which we show is important for myoblast fusion during the growth and maturation process of skeletal muscle. Together, these data broaden our understanding of NF-kappaB during development by showing that in addition to its role as a negative regulator of myogenesis, NF-kappaB also regulates nitric-oxide synthase expression within stromal fibroblasts to stimulate myoblast fusion and muscle hypertrophy.
Figures
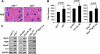
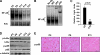


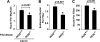
References
-
- Baeuerle P. A., Baltimore D. (1996) Cell 87, 13–20 - PubMed
-
- Baldwin A. S., Jr. (1996) Annu. Rev. Immunol. 14, 649–683 - PubMed
-
- Verma I. M., Stevenson J. K., Schwarz E. M., Van Antwerp D., Miyamoto S. (1995) Genes Dev. 9, 2723–2735 - PubMed
-
- Hayden M. S., Ghosh S. (2004) Genes Dev. 18, 2195–2224 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

