Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling
- PMID: 20018870
- PMCID: PMC2820795
- DOI: 10.1074/jbc.M109.066456
Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling
Abstract
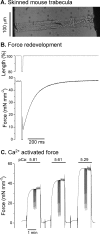
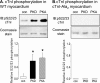
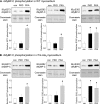
Protein kinase D (PKD), a serine/threonine kinase with emerging cardiovascular functions, phosphorylates cardiac troponin I (cTnI) at Ser(22)/Ser(23), reduces myofilament Ca(2+) sensitivity, and accelerates cross-bridge cycle kinetics. Whether PKD regulates cardiac myofilament function entirely through cTnI phosphorylation at Ser(22)/Ser(23) remains to be established. To determine the role of cTnI phosphorylation at Ser(22)/Ser(23) in PKD-mediated regulation of cardiac myofilament function, we used transgenic mice that express cTnI in which Ser(22)/Ser(23) are substituted by nonphosphorylatable Ala (cTnI-Ala(2)). In skinned myocardium from wild-type (WT) mice, PKD increased cTnI phosphorylation at Ser(22)/Ser(23) and decreased the Ca(2+) sensitivity of force. In contrast, PKD had no effect on the Ca(2+) sensitivity of force in myocardium from cTnI-Ala(2) mice, in which Ser(22)/Ser(23) were unavailable for phosphorylation. Surprisingly, PKD accelerated cross-bridge cycle kinetics similarly in myocardium from WT and cTnI-Ala(2) mice. Because cardiac myosin-binding protein C (cMyBP-C) phosphorylation underlies cAMP-dependent protein kinase (PKA)-mediated acceleration of cross-bridge cycle kinetics, we explored whether PKD phosphorylates cMyBP-C at its PKA sites, using recombinant C1C2 fragments with or without site-specific Ser/Ala substitutions. Kinase assays confirmed that PKA phosphorylates Ser(273), Ser(282), and Ser(302), and revealed that PKD phosphorylates only Ser(302). Furthermore, PKD phosphorylated Ser(302) selectively and to a similar extent in native cMyBP-C of skinned myocardium from WT and cTnI-Ala(2) mice, and this phosphorylation occurred throughout the C-zones of sarcomeric A-bands. In conclusion, PKD reduces myofilament Ca(2+) sensitivity through cTnI phosphorylation at Ser(22)/Ser(23) but accelerates cross-bridge cycle kinetics by a distinct mechanism. PKD phosphorylates cMyBP-C at Ser(302), which may mediate the latter effect.
Figures

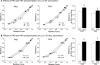


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

