Sulfation of colonic mucins by N-acetylglucosamine 6-O-sulfotransferase-2 and its protective function in experimental colitis in mice
- PMID: 20018871
- PMCID: PMC2825469
- DOI: 10.1074/jbc.M109.067082
Sulfation of colonic mucins by N-acetylglucosamine 6-O-sulfotransferase-2 and its protective function in experimental colitis in mice
Abstract
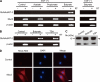
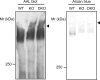
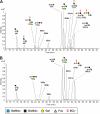
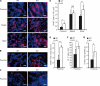
N-Acetylglucosamine 6-O-sulfotransferase-2 (GlcNAc6ST-2) catalyzes the sulfation of mucin-like glycoproteins, which function as ligands for a lymphocyte homing receptor, L-selectin, in the lymph node high endothelial venules (HEVs). We previously showed that GlcNAc6ST-2 is expressed not only in lymph node HEVs but also in the colonic epithelial cells in mice. Here we investigated the regulatory mechanism and physiological significance of colonic expression of GlcNAc6ST-2 in mice. Treatment of a mouse colonic epithelial cell line with butyrate, a short-chain fatty acid produced by anaerobic bacteria, induced GlcNAc6ST-2 expression in the presence of epidermal growth factor. Administration of butyrate in the drinking water stimulated GlcNAc6ST-2 expression in the mouse intestine, indicating that butyrate could serve as a regulatory molecule for the GlcNAc6ST-2 expression in vivo. Immunohistochemical analysis indicated that the sulfation of colonic mucins was greatly diminished in GlcNAc6ST-2-deficient mice. Liquid chromatography coupled to electrospray ionization tandem mass spectrometry of the colonic-mucin O-glycans from wild-type and GlcNAc6ST-2-deficient mice showed that GlcNAc-6-O-sulfation was the predominant sulfate modification of these mucins, and it was exclusively mediated by GlcNAc6ST-2. After colitis induction by dextran sulfate sodium, significantly more leukocyte infiltration was observed in the colon of GlcNAc6ST-2-deficient mice than in that of wild-type mice, indicating that the sulfation of colonic mucins by GlcNAc6ST-2 has a protective function in experimental colitis. These findings indicate that GlcNAc6ST-2, whose expression is regulated by butyrate, is a major sulfotransferase in the biosynthesis of sulfomucins in the mouse colon, where they serve as a mucosal barrier against colonic inflammation.
Figures





References
-
- Bishop J. R., Schuksz M., Esko J. D. (2007) Nature 446, 1030–1037 - PubMed
-
- Honke K., Zhang Y., Cheng X., Kotani N., Taniguchi N. (2004) Glycoconj. J. 21, 59–62 - PubMed
-
- Kawashima H. (2006) Biol. Pharm. Bull. 29, 2343–2349 - PubMed
-
- Fukuda M., Hiraoka N., Akama T. O., Fukuda M. N. (2001) J. Biol. Chem. 276, 47747–47750 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

