Slow onset inhibition of bacterial beta-ketoacyl-acyl carrier protein synthases by thiolactomycin
- PMID: 20018879
- PMCID: PMC2825411
- DOI: 10.1074/jbc.M109.077909
Slow onset inhibition of bacterial beta-ketoacyl-acyl carrier protein synthases by thiolactomycin
Abstract
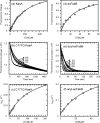
Thiolactomycin (TLM), a natural product thiolactone antibiotic produced by species of Nocardia and Streptomyces, is an inhibitor of the beta-ketoacyl-acyl carrier protein synthase (KAS) enzymes in the bacterial fatty acid synthase pathway. Using enzyme kinetics and direct binding studies, TLM has been shown to bind preferentially to the acyl-enzyme intermediates of the KASI and KASII enzymes from Mycobacterium tuberculosis and Escherichia coli. These studies, which utilized acyl-enzyme mimics in which the active site cysteine was replaced by a glutamine, also revealed that TLM is a slow onset inhibitor of the KASI enzymes KasA and ecFabB but not of the KASII enzymes KasB and ecFabF. The differential affinity of TLM for the acyl-KAS enzymes is proposed to result from structural change involving the movement of helices alpha5 and alpha6 that prepare the enzyme to bind malonyl-AcpM or TLM and that is initiated by formation of hydrogen bonds between the acyl-enzyme thioester and the oxyanion hole. The finding that TLM is a slow onset inhibitor of ecFabB supports the proposal that the long residence time of TLM on the ecFabB homologues in Serratia marcescens and Klebsiella pneumonia is an important factor for the in vivo antibacterial activity of TLM against these two organisms despite the fact that the in vitro MIC values are only 100-200 microg/ml. The mechanistic data on the interaction of TLM with KasA will provide an important foundation for the rational development of high affinity KasA inhibitors based on the thiolactone skeleton.
Figures
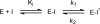
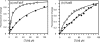
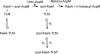
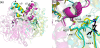
Similar articles
-
Substrate recognition by β-ketoacyl-ACP synthases.Biochemistry. 2011 Dec 13;50(49):10678-86. doi: 10.1021/bi201199x. Epub 2011 Nov 17. Biochemistry. 2011. PMID: 22017312 Free PMC article.
-
Purification and biochemical characterization of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthases KasA and KasB.J Biol Chem. 2001 Dec 14;276(50):47029-37. doi: 10.1074/jbc.M108903200. Epub 2001 Oct 12. J Biol Chem. 2001. PMID: 11600501
-
Thiolactomycin-based β-ketoacyl-AcpM synthase A (KasA) inhibitors: fragment-based inhibitor discovery using transient one-dimensional nuclear overhauser effect NMR spectroscopy.J Biol Chem. 2013 Mar 1;288(9):6045-52. doi: 10.1074/jbc.M112.414516. Epub 2013 Jan 10. J Biol Chem. 2013. PMID: 23306195 Free PMC article.
-
Bacterial beta-ketoacyl-acyl carrier protein synthases as targets for antibacterial agents.Curr Protein Pept Sci. 2003 Feb;4(1):21-9. doi: 10.2174/1389203033380377. Curr Protein Pept Sci. 2003. PMID: 12570782 Review.
-
The β-ketoacyl-ACP synthase from Mycobacterium tuberculosis as potential drug targets.Curr Med Chem. 2011;18(9):1318-24. doi: 10.2174/092986711795029636. Curr Med Chem. 2011. PMID: 21370994 Review.
Cited by
-
Activity Mapping the Acyl Carrier Protein: Elongating Ketosynthase Interaction in Fatty Acid Biosynthesis.Biochemistry. 2020 Sep 29;59(38):3626-3638. doi: 10.1021/acs.biochem.0c00605. Epub 2020 Sep 11. Biochemistry. 2020. PMID: 32857494 Free PMC article.
-
Thiolactomycin-Based Inhibitors of Bacterial β-Ketoacyl-ACP Synthases with in Vivo Activity.J Med Chem. 2016 Jun 9;59(11):5377-90. doi: 10.1021/acs.jmedchem.6b00236. Epub 2016 May 24. J Med Chem. 2016. PMID: 27187871 Free PMC article.
-
Minimization of the Thiolactomycin Biosynthetic Pathway Reveals that the Cytochrome P450 Enzyme TlmF Is Required for Five-Membered Thiolactone Ring Formation.Chembiochem. 2017 Jun 19;18(12):1072-1076. doi: 10.1002/cbic.201700090. Epub 2017 May 11. Chembiochem. 2017. PMID: 28393452 Free PMC article.
-
Mycobacterium tuberculosis: Pathogenesis and therapeutic targets.MedComm (2020). 2023 Sep 4;4(5):e353. doi: 10.1002/mco2.353. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37674971 Free PMC article. Review.
-
A genomics-led approach to deciphering the mechanism of thiotetronate antibiotic biosynthesis.Chem Sci. 2016 Jan 1;7(1):376-385. doi: 10.1039/c5sc03059e. Epub 2015 Oct 8. Chem Sci. 2016. PMID: 28791099 Free PMC article.
References
-
- Bloom B. R., Murray C. J. (1992) Science 257, 1055–1064 - PubMed
-
- DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA
-
- Walsh C. T. (2003) Antibiotics: Actions, Origins, Resistance, pp. 23–49, American Society for Microbiology, Washington, DC
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

