Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects
- PMID: 20018898
- PMCID: PMC2836041
- DOI: 10.1074/jbc.M109.066092
Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects
Abstract
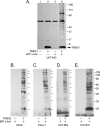
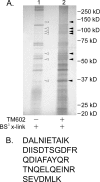
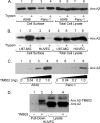
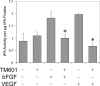
TM601 is a synthetic form of chlorotoxin, a 36-amino acid peptide derived from the venom of the Israeli scorpion, Leirius quinquestriatus, initially found to specifically bind and inhibit the migration of glioma cells in culture. Subsequent studies demonstrated specific in vitro binding to additional tumor cell lines. Recently, we demonstrated that proliferating human vascular endothelial cells are the only normal cell line tested that exhibits specific binding to TM601. Here, we identify annexin A2 as a novel binding partner for TM601 in multiple human tumor cell lines and human umbilical vein endothelial cell (HUVEC). We demonstrate that the surface binding of TM601 to the pancreatic tumor cell line Panc-1 is dependent on the expression of annexin A2. Identification of annexin A2 as a binding partner for TM601 is also consistent with the anti-angiogenic effects of TM601. Annexin A2 functions in angiogenesis by binding to tissue plasminogen activator and regulating plasminogen activation on vascular endothelial cells. We demonstrate that in HUVECs, TM601 inhibits both vascular endothelial growth factor- and basic fibroblast growth factor-induced tissue plasminogen activator activation, which is required for activation of plasminogen to plasmin. Consistent with inhibition of cell surface protease activity, TM601 also inhibits platelet-derived growth factor-C induced trans-well migration of both HUVEC and U373-MG glioma cells.
Figures


References
-
- DeBin J. A., Maggio J. E., Strichartz G. R. (1993) Am. J. Physiol. 264, C361–C369 - PubMed
-
- Mamelak A. N., Jacoby D. B. (2007) Expert Opin. Drug Deliv. 4, 175–186 - PubMed
-
- Mamelak A. N., Rosenfeld S., Bucholz R., Raubitschek A., Nabors L. B., Fiveash J. B., Shen S., Khazaeli M. B., Colcher D., Liu A., Osman M., Guthrie B., Schade-Bijur S., Hablitz D. M., Alvarez V. L., Gonda M. A. (2006) J. Clin. Oncol. 24, 3644–3650 - PubMed
-
- Gibbin T. E., Senzer N., Raizer J. J., Shen J., Nabors L. B., Wiranowska M., Fiveash J. B. (2009) J. Clin. Oncol. 27, e14507
-
- Ullrich N., Gillespie G. Y., Sontheimer H. (1995) Neuroreport 7, 343–347 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

