Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry
- PMID: 20018901
- PMCID: PMC2826649
- DOI: 10.1093/jxb/erp370
Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry
Abstract
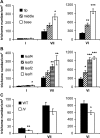
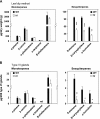
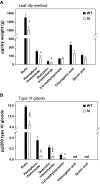
Trichomes are specialized epidermal structures that function as physical and chemical deterrents against arthropod herbivores. Aerial tissues of cultivated tomato (Solanum lycopersicum) are populated by several morphologically distinct trichome types, the most abundant of which is the type VI glandular trichome that produces various specialized metabolites. Here, the effect of the hairless (hl) mutation on trichome density and morphology, chemical composition, and resistance to a natural insect herbivore of tomato was investigated. The results show that the major effect of hl on pubescence results from structural distortion (bending and swelling) of all trichome types in aerial tissues. Leaf surface extracts and isolated type VI glands from hl plants contained wild-type levels of monoterpenes, glycoalkaloids, and acyl sugars, but were deficient in sesquiterpene and polyphenolic compounds implicated in anti-insect defence. No-choice bioassays showed that hl plants are compromised in resistance to the specialist herbivore Manduca sexta. These results establish a link between the morphology and chemical composition of glandular trichomes in cultivated tomato, and show that hl-mediated changes in these leaf surface traits correlate with decreased resistance to insect herbivory.
Figures





References
-
- Ahlstrand G. Low-temperature low-voltage scanning microscopy (LTLVSEM) of uncoated frozen biological materials: a simple alternative. In: Bailey G, Corbett J, Dimlich R, Michael J, Zaluzec N, editors. Proceedings of Microscopy Microanalysis. San Francisco, CA: San Francisco Press; 1996. 918.
-
- Antonious GF. Production and quantification of methyl ketones in wild tomato accessions. Journal of Environmental Science and Health Part B. 2001;36:835–848. - PubMed
-
- Antonious GF, Snyder JC. Natural products: repellency and toxicity of wild tomato leaf extracts to the two-spotted spider mite, Tetranychus urticae Koch. Journal of Environmental Science and Health Part B. 2006;41:43–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

