A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model
- PMID: 20018916
- PMCID: PMC2830759
- DOI: 10.1182/blood-2009-04-215855
A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model
Abstract
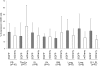
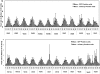
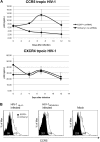
Inhibiting the expression of the HIV-1 coreceptor CCR5 holds great promise for controlling HIV-1 infection in patients. Here we report stable knockdown of human CCR5 by a short hairpin RNA (shRNA) in a humanized bone marrow/liver/thymus (BLT) mouse model. We delivered a potent shRNA against CCR5 into human fetal liver-derived CD34(+) hematopoietic progenitor/stem cells (HPSCs) by lentiviral vector transduction. We transplanted vector-transduced HPSCs solidified with Matrigel and a thymus segment under the mouse kidney capsule. Vector-transduced autologous CD34(+) cells were subsequently injected in the irradiated mouse, intended to create systemic reconstitution. CCR5 expression was down-regulated in human T cells and monocytes/macrophages in systemic lymphoid tissues, including gut-associated lymphoid tissue, the major site of HIV-1 replication. The shRNA-mediated CCR5 knockdown had no apparent adverse effects on T-cell development as assessed by polyclonal T-cell receptor Vbeta family development and naive/memory T-cell differentiation. CCR5 knockdown in the secondary transplanted mice suggested the potential of long-term hematopoietic reconstitution by the shRNA-transduced HPSCs. CCR5 tropic HIV-1 infection was effectively inhibited in mouse-derived human splenocytes ex vivo. These results demonstrate that lentiviral vector delivery of shRNA into human HPSCs could stably down-regulate CCR5 in systemic lymphoid organs in vivo.
Figures






Similar articles
-
Engineering HIV-1-resistant T-cells from short-hairpin RNA-expressing hematopoietic stem/progenitor cells in humanized BLT mice.PLoS One. 2012;7(12):e53492. doi: 10.1371/journal.pone.0053492. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300932 Free PMC article.
-
Inhibition of HIV-1 infection by a unique short hairpin RNA to chemokine receptor 5 delivered into macrophages through hematopoietic progenitor cell transduction.J Gene Med. 2010 Mar;12(3):255-65. doi: 10.1002/jgm.1440. J Gene Med. 2010. PMID: 20186995 Free PMC article.
-
Generation of an HIV-1-resistant immune system with CD34(+) hematopoietic stem cells transduced with a triple-combination anti-HIV lentiviral vector.J Virol. 2012 May;86(10):5719-29. doi: 10.1128/JVI.06300-11. Epub 2012 Mar 7. J Virol. 2012. PMID: 22398281 Free PMC article.
-
CCR5-targeted hematopoietic stem cell gene approaches for HIV disease: current progress and future prospects.Curr Stem Cell Res Ther. 2012 Jul;7(4):310-7. doi: 10.2174/157488812800793108. Curr Stem Cell Res Ther. 2012. PMID: 22486585 Review.
-
Chemokine receptor 5 knockout strategies.Curr Opin HIV AIDS. 2011 Jan;6(1):74-9. doi: 10.1097/COH.0b013e32834122d7. Curr Opin HIV AIDS. 2011. PMID: 21242897 Free PMC article. Review.
Cited by
-
Application of Animal Models in Cancer Research: Recent Progress and Future Prospects.Cancer Manag Res. 2021 Mar 15;13:2455-2475. doi: 10.2147/CMAR.S302565. eCollection 2021. Cancer Manag Res. 2021. PMID: 33758544 Free PMC article. Review.
-
Utilizing Adenovirus Knob Proteins as Carriers in Cancer Gene Therapy Amidst the Presence of Anti-Knob Antibodies.Int J Mol Sci. 2024 Oct 3;25(19):10679. doi: 10.3390/ijms251910679. Int J Mol Sci. 2024. PMID: 39409008 Free PMC article.
-
Augmentation of IFN-γ by bone marrow derived immune cells in the presence of severe suppression of IFN-γ in gingivae induced by zoledronic acid and denosumab in Hu-BLT mice model of ONJ.Front Endocrinol (Lausanne). 2023 Jan 20;14:1111627. doi: 10.3389/fendo.2023.1111627. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36742414 Free PMC article.
-
Pre-clinical modeling of CCR5 knockout in human hematopoietic stem cells by zinc finger nucleases using humanized mice.J Infect Dis. 2013 Nov;208 Suppl 2(Suppl 2):S160-4. doi: 10.1093/infdis/jit382. J Infect Dis. 2013. PMID: 24151324 Free PMC article. Review.
-
A Simple Mouse Model for the Study of Human Immunodeficiency Virus.AIDS Res Hum Retroviruses. 2016 Feb;32(2):194-202. doi: 10.1089/AID.2015.0211. Epub 2015 Dec 17. AIDS Res Hum Retroviruses. 2016. PMID: 26564392 Free PMC article.
References
-
- Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9(4):551–562. - PubMed
-
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. - PubMed
-
- Mosier DE. Virus and target cell evolution in human immunodeficiency virus type 1 infection. Immunol Res. 2000;21(2):253–258. - PubMed
-
- Simmons G, Reeves JD, Hibbitts S, et al. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol Rev. 2000;177:112–126. - PubMed
-
- Ioannidis JP, Rosenberg PS, Goedert JJ, et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3′A alleles on HIV-1 disease progression: an international meta-analysis of individual-patient data. Ann Intern Med. 2001;135(9):782–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

