High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up
- PMID: 20018919
- PMCID: PMC2844020
- DOI: 10.1182/blood-2009-06-225375
High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up
Abstract
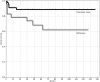
Severe aplastic anemia (SAA) is a life-threatening bone marrow failure disorder that can be treated with bone marrow transplantation, immunosuppressive therapy, and high-dose cyclophosphamide. Here, we report long-term follow-up on 67 SAA patients (44 treatment-naive and 23 refractory) treated with high-dose cyclophosphamide. At 10 years, the overall actuarial survival was 88%, the response rate was 71% with the majority being complete, and the actuarial event-free survival was 58% in 44 treatment-naive SAA patients. Patients with refractory SAA fared less well after high-dose cyclophosphamide therapy; at 10 years, overall actuarial survival, response, and actuarial event-free survival rates were 62%, 48%, and 27%, respectively. High-dose cyclophosphamide is highly effective therapy for severe aplastic anemia. Large randomized controlled trials will be necessary to establish how results of high-dose cyclophosphamide compare with either bone marrow transplantation or standard immunosuppressive regimens, such as antithymocyte globulin and cyclosporine.
Figures

Comment in
-
Cyclophosphamide in aplastic anemia?Blood. 2010 Mar 18;115(11):2120-1. doi: 10.1182/blood-2010-01-261362. Blood. 2010. PMID: 20299517 No abstract available.
Similar articles
-
Complete remission in severe aplastic anemia after high-dose cyclophosphamide without bone marrow transplantation.Blood. 1996 Jan 15;87(2):491-4. Blood. 1996. PMID: 8555470 Clinical Trial.
-
High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases.Medicine (Baltimore). 2011 Mar;90(2):89-98. doi: 10.1097/MD.0b013e318210e685. Medicine (Baltimore). 2011. PMID: 21358440 Free PMC article. Clinical Trial.
-
Durable treatment-free remission after high-dose cyclophosphamide therapy for previously untreated severe aplastic anemia.Ann Intern Med. 2001 Oct 2;135(7):477-83. doi: 10.7326/0003-4819-135-7-200110020-00006. Ann Intern Med. 2001. PMID: 11578150 Clinical Trial.
-
Cyclophosphamide and other new agents for the treatment of severe aplastic anemia.Semin Hematol. 2000 Jan;37(1):102-9. doi: 10.1016/s0037-1963(00)90034-9. Semin Hematol. 2000. PMID: 10676915 Review.
-
Successful engraftment of haploidentical bone marrow with post-transplantation cyclophosphamide in patients with aplastic anemia.Pediatr Transplant. 2020 Mar;24(2):e13652. doi: 10.1111/petr.13652. Epub 2020 Jan 16. Pediatr Transplant. 2020. PMID: 31944531 Review.
Cited by
-
Efficacy of JAK1/2 inhibition in murine immune bone marrow failure.Blood. 2023 Jan 5;141(1):72-89. doi: 10.1182/blood.2022015898. Blood. 2023. PMID: 36130301 Free PMC article.
-
Successful autologous cord blood transplantation in a child with acquired severe aplastic anemia.Pediatr Transplant. 2013 May;17(3):E104-7. doi: 10.1111/petr.12068. Epub 2013 Mar 7. Pediatr Transplant. 2013. PMID: 23464883 Free PMC article.
-
Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients.Clin Infect Dis. 2012 Apr;54(8):1053-63. doi: 10.1093/cid/cir1035. Epub 2012 Mar 12. Clin Infect Dis. 2012. PMID: 22412059 Free PMC article.
-
[Advances in the treatment of severe aplastic anemia].Zhonghua Xue Ye Xue Za Zhi. 2015 Aug;36(8):711-5. doi: 10.3760/cma.j.issn.0253-2727.2015.08.021. Zhonghua Xue Ye Xue Za Zhi. 2015. PMID: 26462649 Free PMC article. Chinese. No abstract available.
-
High-dose cyclophosphamide without stem cell rescue in immune-mediated necrotizing myopathies.Neurol Neuroimmunol Neuroinflamm. 2017 Jul 7;4(5):e381. doi: 10.1212/NXI.0000000000000381. eCollection 2017 Sep. Neurol Neuroimmunol Neuroinflamm. 2017. PMID: 28975138 Free PMC article.
References
-
- Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365(9471):1647–1656. - PubMed
-
- Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101(4):1236–1242. - PubMed
-
- Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–1135. - PubMed
-
- Doney K, Storb R, Buckner CD, et al. Treatment of aplastic anemia with antithymocyte globulin, high-dose corticosteroids, and androgens. Exp Hematol. 1987;15(3):239–242. - PubMed

