Calcium signaling via two-pore channels: local or global, that is the question
- PMID: 20018950
- PMCID: PMC2838574
- DOI: 10.1152/ajpcell.00475.2009
Calcium signaling via two-pore channels: local or global, that is the question
Abstract
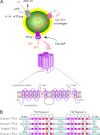
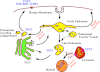
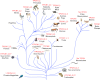
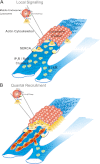
Recently, we identified, for the first time, two-pore channels (TPCs, TPCN for gene name) as a novel family of nicotinic acid adenine dinucleotide phosphate (NAADP)-gated, endolysosome-targeted calcium release channels. Significantly, three subtypes of TPCs have been characterized, TPC1-3, with each being targeted to discrete acidic calcium stores, namely lysosomes (TPC2) and endosomes (TPC1 and TPC3). That TPCs act as NAADP-gated calcium release channels is clear, given that NAADP binds to high- and low-affinity sites associated with TPC2 and thereby induces calcium release and homologous desensitization, as observed in the case of endogenous NAADP receptors. Moreover, NAADP-evoked calcium signals via TPC2 are ablated by short hairpin RNA knockdown of TPC2 and by depletion of acidic calcium stores with bafilomycin. Importantly, however, NAADP-evoked calcium signals were biphasic in nature, with an initial phase of calcium release from lysosomes via TPC2, being subsequently amplified by calcium-induced calcium release (CICR) from the endoplasmic reticulum (ER). In marked contrast, calcium release via endosome-targeted TPC1 induced only spatially restricted calcium signals that were not amplified by CICR from the ER. These findings provide new insights into the mechanisms that cells may utilize to "filter" calcium signals via junctional complexes to determine whether a given signal remains local or is converted into a propagating global signal. Essentially, endosomes and lysosomes represent vesicular calcium stores, quite unlike the ER network, and TPCs do not themselves support CICR or, therefore, propagating regenerative calcium waves. Thus "quantal" vesicular calcium release via TPCs must subsequently recruit inositol 1,4,5-trisphoshpate receptors and/or ryanodine receptors on the ER by CICR to evoke a propagating calcium wave. This may call for a revision of current views on the mechanisms of intracellular calcium signaling. The purpose of this review is, therefore, to provide an appropriate framework for future studies in this area.
Figures


Similar articles
-
Both RyRs and TPCs are required for NAADP-induced intracellular Ca²⁺ release.Cell Calcium. 2015 Sep;58(3):237-45. doi: 10.1016/j.ceca.2015.05.005. Epub 2015 Jun 10. Cell Calcium. 2015. PMID: 26100948 Free PMC article.
-
NAADP mobilizes calcium from acidic organelles through two-pore channels.Nature. 2009 May 28;459(7246):596-600. doi: 10.1038/nature08030. Epub 2009 Apr 22. Nature. 2009. PMID: 19387438 Free PMC article.
-
NAADP-sensitive two-pore channels are present and functional in gastric smooth muscle cells.Cell Calcium. 2014 Aug;56(2):51-8. doi: 10.1016/j.ceca.2014.04.005. Epub 2014 Apr 29. Cell Calcium. 2014. PMID: 24882212
-
Modulation of Calcium Entry by the Endo-lysosomal System.Adv Exp Med Biol. 2016;898:423-47. doi: 10.1007/978-3-319-26974-0_18. Adv Exp Med Biol. 2016. PMID: 27161239 Review.
-
Activation of endo-lysosomal two-pore channels by NAADP and PI(3,5)P2. Five things to know.Cell Calcium. 2022 May;103:102543. doi: 10.1016/j.ceca.2022.102543. Epub 2022 Jan 25. Cell Calcium. 2022. PMID: 35123238 Free PMC article. Review.
Cited by
-
Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH.J Biol Chem. 2013 Aug 16;288(33):24247-63. doi: 10.1074/jbc.M113.484253. Epub 2013 Jul 8. J Biol Chem. 2013. Retraction in: J Biol Chem. 2017 Jul 21;292(29):12088. doi: 10.1074/jbc.A113.484253. PMID: 23836916 Free PMC article. Retracted.
-
Anesthesia-induced developmental neurodegeneration: the role of neuronal organelles.Front Neurol. 2012 Oct 11;3:141. doi: 10.3389/fneur.2012.00141. eCollection 2012. Front Neurol. 2012. PMID: 23087668 Free PMC article.
-
Loss of Two-Pore Channel 2 (TPC2) Expression Increases the Metastatic Traits of Melanoma Cells by a Mechanism Involving the Hippo Signalling Pathway and Store-Operated Calcium Entry.Cancers (Basel). 2020 Aug 24;12(9):2391. doi: 10.3390/cancers12092391. Cancers (Basel). 2020. PMID: 32846966 Free PMC article.
-
TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP.FEBS Lett. 2010 May 17;584(10):1966-74. doi: 10.1016/j.febslet.2010.02.028. Epub 2010 Feb 14. FEBS Lett. 2010. PMID: 20159015 Free PMC article. Review.
-
Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg.J Biol Chem. 2012 Jan 20;287(4):2308-15. doi: 10.1074/jbc.M111.306563. Epub 2011 Nov 23. J Biol Chem. 2012. PMID: 22117077 Free PMC article.
References
-
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. Activation and inactivation of Ca2+ release by NAADP+. J Biol Chem 271: 8513–8516, 1996 - PubMed
-
- Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem 270: 30327–30333, 1995 - PubMed
-
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SR, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci 117: 2813–2825, 2004 - PubMed
-
- Baravalle G, Schober D, Huber M, Bayer N, Murphy RF, Fuchs R. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res 320: 99–113, 2005 - PubMed
-
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol 130: 847–855, 1995 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

