Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: synergistic modulation by cyclic AMP and hyperphosphatemia
- PMID: 20018951
- PMCID: PMC2838579
- DOI: 10.1152/ajpcell.00419.2009
Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: synergistic modulation by cyclic AMP and hyperphosphatemia
Abstract
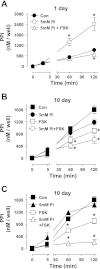
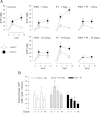
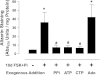
Vascular calcification is a multifaceted process involving gain of calcification inducers and loss of calcification inhibitors. One such inhibitor is inorganic pyrophosphate (PP(i)), and regulated generation and homeostasis of extracellular PP(i) is a critical determinant of soft-tissue mineralization. We recently described an autocrine mechanism of extracellular PP(i) generation in cultured rat aortic vascular smooth muscle cells (VSMC) that involves both ATP release coupled to the ectophosphodiesterase/pyrophosphatase ENPP1 and efflux of intracellular PP(i) mediated or regulated by the plasma membrane protein ANK. We now report that increased cAMP signaling and elevated extracellular inorganic phosphate (P(i)) act synergistically to induce calcification of these VSMC that is correlated with progressive reduction in ability to accumulate extracellular PP(i). Attenuated PP(i) accumulation was mediated in part by cAMP-dependent decrease in ANK expression coordinated with cAMP-dependent increase in expression of TNAP, the tissue nonselective alkaline phosphatase that degrades PP(i). Stimulation of cAMP signaling did not alter ATP release or ENPP1 expression, and the cAMP-induced changes in ANK and TNAP expression were not sufficient to induce calcification. Elevated extracellular P(i) alone elicited only minor calcification and no significant changes in ANK, TNAP, or ENPP1. In contrast, combined with a cAMP stimulus, elevated P(i) induced decreases in the ATP release pathway(s) that supports ENPP1 activity; this resulted in markedly reduced rates of PP(i) accumulation that facilitated robust calcification. Calcified VSMC were characterized by maintained expression of multiple SMC differentiation marker proteins including smooth muscle (SM) alpha-actin, SM22alpha, and calponin. Notably, addition of exogenous ATP (or PP(i) per se) rescued cAMP + phosphate-treated VSMC cultures from progression to the calcified state. These observations support a model in which extracellular PP(i) generation mediated by both ANK- and ATP release-dependent mechanisms serves as a critical regulator of VSMC calcification.
Figures




Similar articles
-
Autocrine ATP release coupled to extracellular pyrophosphate accumulation in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2009 Apr;296(4):C828-39. doi: 10.1152/ajpcell.00619.2008. Epub 2009 Feb 4. Am J Physiol Cell Physiol. 2009. PMID: 19193865 Free PMC article.
-
Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle.Am J Physiol Heart Circ Physiol. 2011 Jul;301(1):H61-8. doi: 10.1152/ajpheart.01020.2010. Epub 2011 Apr 13. Am J Physiol Heart Circ Physiol. 2011. PMID: 21490328 Free PMC article.
-
Elevated glucose levels increase vascular calcification risk by disrupting extracellular pyrophosphate metabolism.Cardiovasc Diabetol. 2024 Nov 11;23(1):405. doi: 10.1186/s12933-024-02502-w. Cardiovasc Diabetol. 2024. PMID: 39529124 Free PMC article.
-
Inorganic pyrophosphate generation and disposition in pathophysiology.Am J Physiol Cell Physiol. 2001 Jul;281(1):C1-C11. doi: 10.1152/ajpcell.2001.281.1.C1. Am J Physiol Cell Physiol. 2001. PMID: 11401820 Review.
-
Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia.Cell Mol Life Sci. 2019 Jun;76(11):2077-2091. doi: 10.1007/s00018-019-03054-z. Epub 2019 Mar 18. Cell Mol Life Sci. 2019. PMID: 30887097 Free PMC article. Review.
Cited by
-
Regulatory circuits controlling vascular cell calcification.Cell Mol Life Sci. 2013 Sep;70(17):3187-97. doi: 10.1007/s00018-012-1231-y. Epub 2012 Dec 27. Cell Mol Life Sci. 2013. PMID: 23269436 Free PMC article. Review.
-
Discoidin Domain Receptor-1 Regulates Calcific Extracellular Vesicle Release in Vascular Smooth Muscle Cell Fibrocalcific Response via Transforming Growth Factor-β Signaling.Arterioscler Thromb Vasc Biol. 2016 Mar;36(3):525-33. doi: 10.1161/ATVBAHA.115.307009. Epub 2016 Jan 21. Arterioscler Thromb Vasc Biol. 2016. PMID: 26800565 Free PMC article.
-
Activation of nuclear factor-kappa B by TNF promotes nucleus pulposus mineralization through inhibition of ANKH and ENPP1.Sci Rep. 2021 Apr 15;11(1):8271. doi: 10.1038/s41598-021-87665-2. Sci Rep. 2021. PMID: 33859255 Free PMC article.
-
RNA-seq analysis of extracellular vesicles from hyperphosphatemia-stimulated endothelial cells provides insight into the mechanism underlying vascular calcification.BMC Nephrol. 2022 May 21;23(1):192. doi: 10.1186/s12882-022-02823-6. BMC Nephrol. 2022. PMID: 35597927 Free PMC article.
-
Reduction of inorganic phosphate-induced human smooth muscle cells calcification by inhibition of protein kinase A and p38 mitogen-activated protein kinase.Heart Vessels. 2014 Sep;29(5):718-22. doi: 10.1007/s00380-013-0427-x. Epub 2013 Oct 19. Heart Vessels. 2014. PMID: 24141990
References
-
- Beck GR., Jr Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem 90: 234–243, 2003 - PubMed
-
- Brown NA, Stofko RE, Uhler MD. Induction of alkaline phosphatase in mouse L cells by overexpression of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 265: 13181–13189, 1990 - PubMed
-
- Diglio CA, Grammas P, Giacomelli F, Wiener J. Angiogenesis in rat aorta ring explant cultures. Lab Invest 60: 523–531, 1989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

