Tetramerization domain mutations in KCNA5 affect channel kinetics and cause abnormal trafficking patterns
- PMID: 20018952
- PMCID: PMC2838577
- DOI: 10.1152/ajpcell.00464.2009
Tetramerization domain mutations in KCNA5 affect channel kinetics and cause abnormal trafficking patterns
Abstract
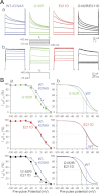
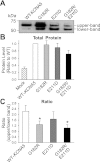
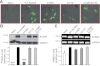
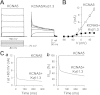
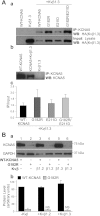
The activity of voltage-gated K(+) (K(V)) channels plays an important role in regulating pulmonary artery smooth muscle cell (PASMC) contraction, proliferation, and apoptosis. The highly conserved NH(2)-terminal tetramerization domain (T1) of K(V) channels is important for proper channel assembly, association with regulatory K(V) beta-subunits, and localization of the channel to the plasma membrane. We recently reported two nonsynonymous mutations (G182R and E211D) in the KCNA5 gene of patients with idiopathic pulmonary arterial hypertension, which localize to the T1 domain of KCNA5. To study the electrophysiological properties and expression patterns of the mutants compared with the wild-type (WT) channel in vitro, we transfected HEK-293 cells with WT KCNA5, G182R, E211D, or the double mutant G182R/E211D channel. The mutants form functional channels; however, whole cell current kinetic differences between WT and mutant channels exist. Steady-state inactivation curves of the G182R and G182R/E211D channels reveal accelerated inactivation; the mutant channels inactivated at more hyperpolarized potentials compared with the WT channel. Channel protein expression was also decreased by the mutations. Compared with the WT channel, which was present in its mature glycosylated form, the mutant channels are present in greater proportion in their immature form in HEK-293 cells. Furthermore, G182R protein level is greatly reduced in COS-1 cells compared with WT. Immunostaining data support the hypothesis that, while WT protein localizes to the plasma membrane, mutant protein is mainly retained in intracellular packets. Overall, these data support a role for the T1 domain in channel kinetics as well as in KCNA5 channel subcellular localization.
Figures




References
-
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 101: 2319–2330, 1998 - PMC - PubMed
-
- Babila T, Moscucci A, Wang H, Weaver FE, Koren G. Assembly of mammalian voltage-gated potassium channels: evidence for an important role of the first transmembrane segment. Neuron 12: 615–626, 1994 - PubMed
-
- Berridge MJ. Calcium signalling and cell proliferation. Bioessays 17: 491–500, 1995 - PubMed
-
- Bixby KA, Nanao MH, Shen NV, Kreusch A, Bellamy H, Pfaffinger PJ, Choe S. Zn2+-binding and molecular determinants of tetramerization in voltage-gated K+ channels. Nat Struct Biol 6: 38–43, 1999 - PubMed
-
- Brevnova EE, Platoshyn O, Zhang S, Yuan JX. Overexpression of human KCNA5 increases IK(V) and enhances apoptosis. Am J Physiol Cell Physiol 287: C715–C722, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

