Novel acyl-coenzyme A:monoacylglycerol acyltransferase plays an important role in hepatic triacylglycerol secretion
- PMID: 20018982
- PMCID: PMC3035505
- DOI: 10.1194/jlr.M002584
Novel acyl-coenzyme A:monoacylglycerol acyltransferase plays an important role in hepatic triacylglycerol secretion
Abstract
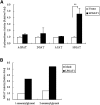
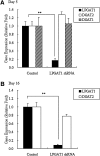
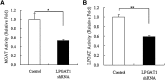
Acyl-CoA:monoacylglycerol acyltransferase (MGAT) plays a predominant role in the resynthesis of triacylglycerol in the small intestine, but its contribution to triacylglycerol synthesis in other tissues, such as the liver, is not clear. In this study, we identified a novel MGAT gene, which is identical with lysophosphatidylglycerol acyltransferase1 (LPGAT1). Mouse LPGAT1 is expressed in a number of tissues and most highly expressed in the liver. Hepatic LPGAT1 expression in diabetic db/db mice is higher than that in the control db/m mouse, which is consistent with increased hepatic MGAT activity in db/db mouse. To elucidate the role of LPGAT1 gene in lipid metabolism in db/db mice, we constructed an adenovirus of short hairpin RNA (shRNA) targeting LPGAT1 to selectively knockdown LPGAT1 gene expression in the liver. Hepatic MGAT activity and LPGAT1 expression in db/db mice infected with LPGAT1 shRNA adenovirus were significantly lower than those in mice infected with the control virus. Notably, treatment with LPGAT1 shRNA adenovirus caused a marked reduction in serum triacylglycerol and cholesterol levels and a significant increase in hepatic cholesterol level. These findings indicate that LPGAT1, a newly identified MGAT enzyme, plays a significant role in hepatic triacylglycerol synthesis and secretion in db/db mice.
Figures






Similar articles
-
Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase.Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):8512-7. doi: 10.1073/pnas.132274899. Epub 2002 Jun 19. Proc Natl Acad Sci U S A. 2002. PMID: 12077311 Free PMC article.
-
The monoacylglycerol acyltransferase pathway contributes to triacylglycerol synthesis in HepG2 cells.Sci Rep. 2022 Mar 23;12(1):4943. doi: 10.1038/s41598-022-08946-y. Sci Rep. 2022. PMID: 35322811 Free PMC article.
-
Identification and characterization of a gene encoding human LPGAT1, an endoplasmic reticulum-associated lysophosphatidylglycerol acyltransferase.J Biol Chem. 2004 Dec 31;279(53):55866-74. doi: 10.1074/jbc.M406710200. Epub 2004 Oct 12. J Biol Chem. 2004. PMID: 15485873
-
Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism.Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E10-8. doi: 10.1152/ajpendo.90949.2008. Epub 2008 Dec 30. Am J Physiol Endocrinol Metab. 2009. PMID: 19116371 Free PMC article. Review.
-
Mechanisms of intestinal triacylglycerol synthesis.Biochim Biophys Acta Mol Cell Biol Lipids. 2022 Jun;1867(6):159151. doi: 10.1016/j.bbalip.2022.159151. Epub 2022 Mar 14. Biochim Biophys Acta Mol Cell Biol Lipids. 2022. PMID: 35296424 Review.
Cited by
-
Genome-wide scan reveals genetic divergence and diverse adaptive selection in Chinese local cattle.BMC Genomics. 2019 Jun 14;20(1):494. doi: 10.1186/s12864-019-5822-y. BMC Genomics. 2019. PMID: 31200634 Free PMC article.
-
Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases.Cancers (Basel). 2024 May 31;16(11):2115. doi: 10.3390/cancers16112115. Cancers (Basel). 2024. PMID: 38893234 Free PMC article. Review.
-
Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver.J Lipid Res. 2012 May;53(5):990-999. doi: 10.1194/jlr.P025536. Epub 2012 Mar 6. J Lipid Res. 2012. PMID: 22394502 Free PMC article.
-
Biological mechanism and functional verification of key genes related to major depressive disorder and type 2 diabetes mellitus.Mamm Genome. 2025 Mar;36(1):66-82. doi: 10.1007/s00335-024-10090-z. Epub 2024 Dec 10. Mamm Genome. 2025. PMID: 39656235
-
Glycerophosphate/Acylglycerophosphate acyltransferases.Biology (Basel). 2014 Nov 19;3(4):801-30. doi: 10.3390/biology3040801. Biology (Basel). 2014. PMID: 25415055 Free PMC article. Review.
References
-
- Coleman R. A., Lee D. P. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43: 134–176. - PubMed
-
- Bell R. M., Coleman R. A. 1980. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 49: 459–487. - PubMed
-
- Manganaro F., Kuksis A. 1985. Purification and preliminary characterization of 2-monoacylglycerol acyltransferase from rat intestinal villus cells. Can. J. Biochem. Cell Biol. 63: 341–347. - PubMed
-
- Bhat B. G., Bardes E. S-G., Coleman R. A. 1993. Solubilization and partial purification of neonatally expressed rat hepatic microsomal monoacylglycerol acyltransferase. Arch. Biochem. Biophys. 300: 663–669. - PubMed
-
- Bhat B. G., Wang P., Coleman R. A. 1994. Hepatic monoacylglycerol acyltransferase is regulated by sn-1,2-diacylglycerol and by specific lipids in Triton X-100/phospholipid-mixed micelles. J. Biol. Chem. 269: 13172–13178. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

