Red Mold Rice Mitigates Oral Carcinogenesis in 7,12-Dimethyl-1,2-Benz[a]anthracene-Induced Oral Carcinogenesis in Hamster
- PMID: 20019075
- PMCID: PMC3095506
- DOI: 10.1093/ecam/nep215
Red Mold Rice Mitigates Oral Carcinogenesis in 7,12-Dimethyl-1,2-Benz[a]anthracene-Induced Oral Carcinogenesis in Hamster
Abstract
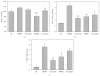
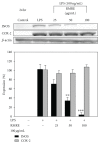
The prevalence of oral tumor has exponentially increased in recent years; however, the effective therapies or prevention strategies are not sufficient. Red mold rice is a traditional Chinese food, and several reports have demonstrated that red mold rice had an anti-tumor effect. However, the possible anti-tumor mechanisms of the red mold rice are unclear. In this study, we examined the anti-tumor effect of red mold rice on 7,12-dimethyl-1,2-benz[a]anthracene (DMBA)-induced oral tumor in hamster. The ethanol extract of red mold rice (RMRE) treatment significantly decreases the levels of DMBA-induced reactive oxygen species, nitro oxide and prostaglandin E(2) than those of the lovastatin-treated group (P < .001). Moreover, RMRE decreases the formation of oral tumor induced by DMBA. Monacolin K, monascin, ankaflavin or other red mold rice metabolites had been reported to decrease inflammation and oxidative stress and exerted anti-tumor effects. Therefore, we evaluated the anti-inflammation and anti-oxidative stress effects of monacolin K, monascin, ankaflavin and citrinin in lipopolysaccharide-treated RAW264.7 cells. We found that RMRE reduced the LPS-induced nitrite levels in RAW264.7 cells better than monacolin K, monascin, ankaflavin or citrinin (P < .05).
Figures

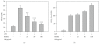
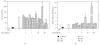
Similar articles
-
Investigation of monacolin K, yellow pigments, and citrinin production capabilities of Monascus purpureus and Monascus ruber (Monascus pilosus).J Food Drug Anal. 2023 Mar 15;31(1):85-94. doi: 10.38212/2224-6614.3438. J Food Drug Anal. 2023. PMID: 37224553 Free PMC article.
-
Monascin and ankaflavin have more anti-atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than monacolin K under the same dosages.J Agric Food Chem. 2013 Jan 9;61(1):143-50. doi: 10.1021/jf304346r. Epub 2012 Dec 24. J Agric Food Chem. 2013. PMID: 23237237
-
Synchronous high-performance liquid chromatography with a photodiode array detector and mass spectrometry for the determination of citrinin, monascin, ankaflavin, and the lactone and acid forms of monacolin K in red mold rice.J AOAC Int. 2011 Jan-Feb;94(1):179-90. J AOAC Int. 2011. PMID: 21391495
-
[Suggestions for revision of quality standards of red yeast rice for medicinal and edible purposes in China].Zhongguo Zhong Yao Za Zhi. 2024 Oct;49(20):5659-5673. doi: 10.19540/j.cnki.cjcmm.20240715.601. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39701748 Review. Chinese.
-
Functional food red yeast rice (RYR) for metabolic syndrome amelioration: a review on pros and cons.World J Microbiol Biotechnol. 2016 May;32(5):87. doi: 10.1007/s11274-016-2035-2. Epub 2016 Apr 2. World J Microbiol Biotechnol. 2016. PMID: 27038957 Review.
Cited by
-
Induction of apoptosis in human breast adenocarcinoma cells MCF-7 by monapurpyridine A, a new azaphilone derivative from Monascus purpureus NTU 568.Molecules. 2012 Jan 11;17(1):664-73. doi: 10.3390/molecules17010664. Molecules. 2012. PMID: 22237681 Free PMC article.
-
Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review.Molecules. 2018 Jan 3;23(1):98. doi: 10.3390/molecules23010098. Molecules. 2018. PMID: 29301350 Free PMC article. Review.
-
New anti-inflammatory and anti-proliferative constituents from fermented red mold rice Monascus purpureus NTU 568.Molecules. 2010 Nov 3;15(11):7815-24. doi: 10.3390/molecules15117815. Molecules. 2010. PMID: 21060290 Free PMC article.
References
-
- MacKenzie J, Ah-See K, Thakker N, et al. Increasing incidence of oral cancer amongst young persons: what is the aetiology? Oral Oncology. 2000;36(4):387–389. - PubMed
-
- Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. Ca-A Cancer Journal for Clinicians. 2004;54(1):8–29. - PubMed
-
- Ma J, Li Y, Ye Q, et al. Constituents of red yeast rice, a traditional Chinese food and medicine. Journal of Agricultural and Food Chemistry. 2000;48(11):5220–5225. - PubMed
-
- Journoud M, Jones PJH. Red yeast rice: a new hypolipidemic drug. Life Sciences. 2004;74(22):2675–2683. - PubMed
-
- Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. Journal of Antibiotics. 1979;32(8):852–854. - PubMed
LinkOut - more resources
Full Text Sources

