Mammalian target of rapamycin (mTOR) induces proliferation and de-differentiation responses to three coordinate pathophysiologic stimuli (mechanical strain, hypoxia, and extracellular matrix remodeling) in rat bladder smooth muscle
- PMID: 20019183
- PMCID: PMC2797892
- DOI: 10.2353/ajpath.2010.080834
Mammalian target of rapamycin (mTOR) induces proliferation and de-differentiation responses to three coordinate pathophysiologic stimuli (mechanical strain, hypoxia, and extracellular matrix remodeling) in rat bladder smooth muscle
Abstract
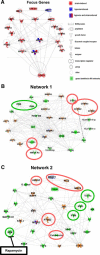
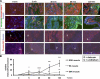
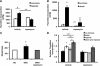
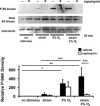
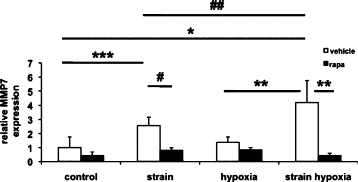
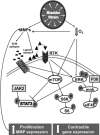
Maladaptive bladder muscle overgrowth and de-differentiation in human bladder obstructive conditions is instigated by coordinate responses to three stimuli: mechanical strain, tissue hypoxia, and extracellular matrix remodeling.( 1,2) Pathway analysis of genes induced by obstructive models of injury in bladder smooth muscle cells (BSMCs) identified a mammalian target of rapamycin (mTOR)-specific inhibitor as a potential pharmacological inhibitor. Strain-induced mTOR-specific S6K activation segregated differently from ERK1/2 activation in intact bladder ex vivo. Though rapamycin's antiproliferative effects in vascular smooth muscle cells are well known, its effects on BSMCs were previously unknown. Rapamycin significantly inhibited proliferation of BSMCs in response to mechanical strain, hypoxia, and denatured collagen. Rapamycin inhibited S6K at mTOR-sensitive phosphorylation sites in response to strain and hypoxia. Rapamycin also supported smooth muscle actin expression in response to strain or hypoxia-induced de-differentiation. Importantly, strain plus hypoxia synergistically augmented mTOR-dependent S6K activation, Mmp7 expression and proliferation. Forced expression of wild-type and constitutively active S6K resulted in loss of smooth muscle actin expression. Decreased smooth muscle actin, increased Mmp7 levels and mTOR pathway activation during in vivo partial bladder obstruction paralleled our in vitro studies. These results point to a coordinate role for mTOR in BSMCs responses to the three stimuli and a potential new therapeutic target for myopathic bladder disease.
Figures



References
-
- Mattiasson A, Uvelius B. Changes in contractile properties in hypertrophic rat urinary bladder. J Urol. 1982;128:1340–1342. - PubMed
-
- Becker A, Baum M. Obstructive uropathy. Early Hum Dev. 2006;82:15–22. - PubMed
-
- Austin JC, Chacko SK, DiSanto M, Canning DA, Zderic SA. A male murine model of partial bladder outlet obstruction reveals changes in detrusor morphology, contractility and Myosin isoform expression. J Urol. 2004;172:1524–1528. - PubMed
-
- Buttyan R, Chen MW, Levin RM. Animal models of bladder outlet obstruction and molecular insights into the basis for the development of bladder dysfunction. Eur Urol. 1997;32 Suppl 1:32–39. - PubMed
-
- Johansson R, Persson K. Phenotypic modulation of cultured bladder smooth muscle cells and the expression of inducible nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2004;286:R642–R648. - PubMed
Uncited reference
-
- Locatelli F, Roger S. Comparative testing and pharmacovigilance of biosimilars. Nephrol, Dial, Transplant. 2006;21 Suppl 5:v13–v16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

