Aberrant inflammatory response to Streptococcus pyogenes in mice lacking myeloid differentiation factor 88
- PMID: 20019195
- PMCID: PMC2808082
- DOI: 10.2353/ajpath.2010.090422
Aberrant inflammatory response to Streptococcus pyogenes in mice lacking myeloid differentiation factor 88
Abstract
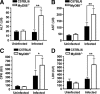
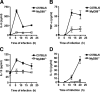
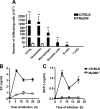
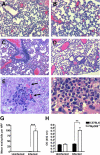
Several in vitro studies have emphasized the importance of toll-like receptor/myeloid differentiation factor 88 (MyD88) signaling in the inflammatory response to Streptococcus pyogenes. Since the extent of inflammation has been implicated in the severity of streptococcal diseases, we have examined here the role of toll-like receptor/MyD88 signaling in the pathophysiology of experimental S. pyogenes infection. To this end, we compared the response of MyD88-knockout (MyD88(-/-)) after subcutaneous inoculation with S. pyogenes with that of C57BL/6 mice. Our results show that MyD88(-/-) mice harbored significantly more bacteria in the organs and succumbed to infection much earlier than C57BL/6 animals. Absence of MyD88 resulted in diminished production of inflammatory cytokines such as interleukin-12, interferon-gamma, and tumor necrosis factor-alpha as well as chemoattractants such as monocyte chemotactic protein-1 (MCP-1) and Keratinocyte-derived chemokine (KC), and hampered recruitment of effector cells involved in bacterial clearance (macrophages and neutrophils) to the infection site. Furthermore, MyD88(-/-) but not C57BL/6 mice exhibited a massive infiltration of eosinophils in infected organs, which can be explained by an impaired production of the regulatory chemokines, gamma interferon-induced monokine (MIG/CXCL9) and interferon-induced protein 10 (IP-10/CXCL10), which can inhibit transmigration of eosinophils. Our results indicate that MyD88 signaling targets effector cells to the site of streptococcal infection and prevents extravasation of cells that can induce tissue damage. Therefore, MyD88 signaling may be important for shaping the quality of the inflammatory response elicited during infection to ensure optimal effector functions.
Figures

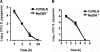
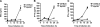
Similar articles
-
Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88.J Immunol. 2003 Dec 1;171(11):6145-53. doi: 10.4049/jimmunol.171.11.6145. J Immunol. 2003. PMID: 14634130
-
Essential role of interleukin-1 signaling in host defenses against group B streptococcus.mBio. 2014 Sep 9;5(5):e01428-14. doi: 10.1128/mBio.01428-14. mBio. 2014. PMID: 25205091 Free PMC article.
-
Immune recognition of Streptococcus pyogenes by dendritic cells.Infect Immun. 2008 Jun;76(6):2785-92. doi: 10.1128/IAI.01680-07. Epub 2008 Apr 7. Infect Immun. 2008. PMID: 18391010 Free PMC article.
-
Streptococcal IdeS and its impact on immune response and inflammation.J Innate Immun. 2012;4(2):132-40. doi: 10.1159/000332940. Epub 2012 Jan 17. J Innate Immun. 2012. PMID: 22248585 Free PMC article. Review.
-
Host responses to group a streptococcus: cell death and inflammation.PLoS Pathog. 2014 Aug 28;10(8):e1004266. doi: 10.1371/journal.ppat.1004266. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25165887 Free PMC article. Review.
Cited by
-
Colonization of the Murine Oropharynx by Streptococcus pyogenes Is Governed by the Rgg2/3 Quorum Sensing System.Infect Immun. 2020 Sep 18;88(10):e00464-20. doi: 10.1128/IAI.00464-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32747598 Free PMC article.
-
Pilus proteins from Streptococcus pyogenes stimulate innate immune responses through Toll-like receptor 2.Immunol Cell Biol. 2022 Mar;100(3):174-185. doi: 10.1111/imcb.12523. Epub 2022 Feb 24. Immunol Cell Biol. 2022. PMID: 35124861 Free PMC article.
-
Dextromethorphan Attenuates NADPH Oxidase-Regulated Glycogen Synthase Kinase 3β and NF-κB Activation and Reduces Nitric Oxide Production in Group A Streptococcal Infection.Antimicrob Agents Chemother. 2018 May 25;62(6):e02045-17. doi: 10.1128/AAC.02045-17. Print 2018 Jun. Antimicrob Agents Chemother. 2018. PMID: 29581121 Free PMC article.
-
The burden of group A Streptococcus (GAS) infections: The challenge continues in the twenty-first century.iScience. 2024 Dec 24;28(1):111677. doi: 10.1016/j.isci.2024.111677. eCollection 2025 Jan 17. iScience. 2024. PMID: 39877071 Free PMC article. Review.
-
Type I interferon production induced by Streptococcus pyogenes-derived nucleic acids is required for host protection.PLoS Pathog. 2011 May;7(5):e1001345. doi: 10.1371/journal.ppat.1001345. Epub 2011 May 19. PLoS Pathog. 2011. PMID: 21625574 Free PMC article.
References
-
- Jaggi P, Shulman ST. Group A streptococcal infections. Pediatr Rev. 2006;27:99–105. - PubMed
-
- Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. - PubMed
-
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. - PubMed
-
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. - PubMed
-
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

