Roles for inflammation and regulatory T cells in colon cancer
- PMID: 20019355
- PMCID: PMC4068028
- DOI: 10.1177/0192623309354110
Roles for inflammation and regulatory T cells in colon cancer
Abstract
Risk for developing cancer rises substantially as a result of poorly regulated inflammatory responses to pathogenic bacterial infections. Anti-inflammatory CD4(+) regulatory cells (T(REG)) function to restore immune homeostasis during chronic inflammatory disorders. It seems logical that T(REG) cells would function to reduce risk of inflammation-associated cancer in the bowel by down-regulating inflammation. It is widely believed, however, that T(REG) function in cancer mainly to suppress protective anticancer inflammatory responses. Thus roles for inflammation, T(REG) cells, and gut bacteria in cancer are paradoxical and are the subject of controversy. Our accumulated data build upon the "hygiene hypothesis" model in which gastrointestinal (GI) infections lead to changes in T(REG) that reduce inflammation-associated diseases. Ability of T(REG) to inhibit or suppress cancer depends upon gut bacteria and IL-10, which serve to maintain immune balance and a protective anti-inflammatory T(REG) phenotype. However, under poorly regulated pro-inflammatory conditions, T(REG) fail to inhibit and may instead contribute to a T helper (Th)-17-driven procarcinogenic process, a cancer state that is reversible by down-regulation of inflammation and interleukin (IL)-6. Consequently, hygienic individuals with a weakened IL-10- and T(REG)-mediated inhibitory loop are highly susceptible to the carcinogenic consequences of elevated inflammation and show more frequent inflammation-associated cancers. Taken together, these data help explain the paradox of inflammation and T(REG) in cancer and indicate that targeted stimulation of T(REG) may promote health and significantly reduce risk of cancer.
Figures
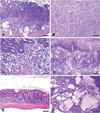
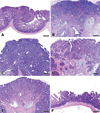


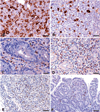

Similar articles
-
Unifying roles for regulatory T cells and inflammation in cancer.Int J Cancer. 2010 Apr 1;126(7):1651-65. doi: 10.1002/ijc.24923. Int J Cancer. 2010. PMID: 19795459 Free PMC article.
-
Cancer inflammation and regulatory T cells.Int J Cancer. 2010 Aug 15;127(4):768-79. doi: 10.1002/ijc.25430. Int J Cancer. 2010. PMID: 20518013 Free PMC article. Review.
-
The inhibitory cytokine IL-35 contributes to regulatory T-cell function.Nature. 2007 Nov 22;450(7169):566-9. doi: 10.1038/nature06306. Nature. 2007. PMID: 18033300
-
Extrathymically generated regulatory T cells control mucosal TH2 inflammation.Nature. 2012 Feb 8;482(7385):395-9. doi: 10.1038/nature10772. Nature. 2012. PMID: 22318520 Free PMC article.
-
Antigen-specific CD4(+) regulatory T cells in the intestine.Inflamm Allergy Drug Targets. 2006 Sep;5(3):191-201. doi: 10.2174/187152806778256043. Inflamm Allergy Drug Targets. 2006. PMID: 16918482 Review.
Cited by
-
Early decrease in postoperative serum albumin predicts severe complications in patients with colorectal cancer after curative laparoscopic surgery.World J Surg Oncol. 2018 Sep 25;16(1):192. doi: 10.1186/s12957-018-1493-4. World J Surg Oncol. 2018. PMID: 30253767 Free PMC article.
-
Hygiene and the world distribution of Alzheimer's disease: Epidemiological evidence for a relationship between microbial environment and age-adjusted disease burden.Evol Med Public Health. 2013 Jan;2013(1):173-86. doi: 10.1093/emph/eot015. Epub 2013 Aug 11. Evol Med Public Health. 2013. PMID: 24481197 Free PMC article.
-
Inflammatory bowel disease-associated colorectal cancer: proctocolectomy and mucosectomy do not necessarily eliminate pouch-related cancer incidences.Int J Colorectal Dis. 2011 May;26(5):533-52. doi: 10.1007/s00384-011-1137-4. Epub 2011 Feb 11. Int J Colorectal Dis. 2011. PMID: 21311893 Free PMC article. Review.
-
Urokinase-type plasminogen activator deficiency promotes neoplasmatogenesis in the colon of mice.Transl Oncol. 2014 Apr;7(2):174-187.e5. doi: 10.1016/j.tranon.2014.02.002. Epub 2014 Mar 4. Transl Oncol. 2014. PMID: 24913672 Free PMC article.
-
Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links.BBA Clin. 2015 Nov 5;5:16-24. doi: 10.1016/j.bbacli.2015.11.002. eCollection 2016 Jun. BBA Clin. 2015. PMID: 27051585 Free PMC article. Review.
References
-
- American Cancer Society (ACS) Cancer facts and figures. Atlanta, GA: U.S. Cancer Fact and Figures. ACS; 2004.
-
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. - PubMed
-
- Badache A, Hynes NE. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 2001;61:383–391. - PubMed
-
- Balkwill F, Coussens LM. Cancer: An inflammatory link. Nature. 2001;431:405–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

