Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal
- PMID: 20019386
- PMCID: PMC2853435
- DOI: 10.1194/jlr.M003673
Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal
Abstract
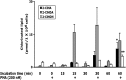
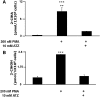
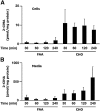
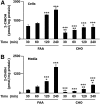
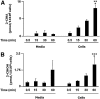
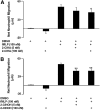
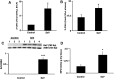
Neutrophils are important in the host response against invading pathogens. One chemical defense mechanism employed by neutrophils involves the production of myeloperoxidase (MPO)-derived HOCl. 2-Chlorohexadecanal (2-ClHDA) is a naturally occurring lipid product of HOCl targeting the vinyl ether bond of plasmalogens. Previous studies have shown that exogenously-added 2-ClHDA is oxidized to 2-chlorohexadecanoic acid (2-ClHA) and reduced to 2-chlorohexadecanol (2-ClHOH) by endothelial cells. These studies show that both 2-ClHA and 2-ClHOH are produced in activated neutrophils in an MPO- and time-dependent manner and are released by neutrophils into media. 2-ClHDA levels peak following 30 min of phorbol 12-myristate-13-acetate stimulation. In contrast, 2-ClHA and 2-ClHOH levels steadily increased over 60 min, suggesting a precursor-product relationship between 2-ClHDA and both 2-ClHA and 2-ClHOH. Additional experiments using wild-type CHO.K1 and CHO.K1 cells deficient in fatty aldehyde dehydrogenase (FALDH), FAA.K1A, demonstrated that 2-ClHDA oxidation to 2-ClHA is dependent on FALDH activity. Furthermore, mice exposed to intranasal Sendai virus displayed lung neutrophil recruitment, as well as elevated 2-ClHA levels in plasma and bronchoalveolar lavage compared with control-treated mice. Taken together, these data demonstrate, for the first time, that metabolites of 2-ClHDA are produced both in vivo as well as in isolated human neutrophils.
Figures
Similar articles
-
Fatty aldehyde and fatty alcohol metabolism: review and importance for epidermal structure and function.Biochim Biophys Acta. 2014 Mar;1841(3):377-89. doi: 10.1016/j.bbalip.2013.09.001. Epub 2013 Sep 12. Biochim Biophys Acta. 2014. PMID: 24036493 Free PMC article. Review.
-
Metabolism of myeloperoxidase-derived 2-chlorohexadecanal.J Biol Chem. 2006 Jun 23;281(25):16849-16860. doi: 10.1074/jbc.M602505200. Epub 2006 Apr 11. J Biol Chem. 2006. PMID: 16611638
-
2-Chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 expression in human coronary artery endothelial cells.Lipids. 2008 Jul;43(7):581-8. doi: 10.1007/s11745-008-3189-y. Epub 2008 May 21. Lipids. 2008. PMID: 18493808 Free PMC article.
-
Strategies for the analysis of chlorinated lipids in biological systems.Free Radic Biol Med. 2013 Jun;59:92-9. doi: 10.1016/j.freeradbiomed.2012.06.013. Epub 2012 Jun 17. Free Radic Biol Med. 2013. PMID: 22713364 Free PMC article.
-
The chlorinated lipidome originating from myeloperoxidase-derived HOCl targeting plasmalogens: Metabolism, clearance, and biological properties.Arch Biochem Biophys. 2018 Mar 1;641:31-38. doi: 10.1016/j.abb.2018.01.010. Epub 2018 Jan 31. Arch Biochem Biophys. 2018. PMID: 29378164 Free PMC article. Review.
Cited by
-
Myeloperoxidase instigates proinflammatory responses in a cecal ligation and puncture rat model of sepsis.Am J Physiol Heart Circ Physiol. 2020 Sep 1;319(3):H705-H721. doi: 10.1152/ajpheart.00440.2020. Epub 2020 Aug 7. Am J Physiol Heart Circ Physiol. 2020. PMID: 32762560 Free PMC article.
-
Characterization of N-Acetyl Cysteine Adducts with Exogenous and Neutrophil-Derived 2-Chlorofatty Aldehyde.Antioxidants (Basel). 2023 Feb 16;12(2):504. doi: 10.3390/antiox12020504. Antioxidants (Basel). 2023. PMID: 36830062 Free PMC article.
-
2-Chlorofatty Aldehyde Elicits Endothelial Cell Activation.Front Physiol. 2020 May 8;11:460. doi: 10.3389/fphys.2020.00460. eCollection 2020. Front Physiol. 2020. PMID: 32457656 Free PMC article.
-
Fatty aldehyde and fatty alcohol metabolism: review and importance for epidermal structure and function.Biochim Biophys Acta. 2014 Mar;1841(3):377-89. doi: 10.1016/j.bbalip.2013.09.001. Epub 2013 Sep 12. Biochim Biophys Acta. 2014. PMID: 24036493 Free PMC article. Review.
-
Identification of glutathione adducts of α-chlorofatty aldehydes produced in activated neutrophils.J Lipid Res. 2015 May;56(5):1014-24. doi: 10.1194/jlr.M058636. Epub 2015 Mar 26. J Lipid Res. 2015. PMID: 25814023 Free PMC article.
References
-
- Klebanoff S. J. 1980. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 93: 480–489. - PubMed
-
- Harrison J. E., Schultz J. 1976. Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem. 251: 1371–1374. - PubMed
-
- Klebanoff S. J., Waltersdorph A. M., Rosen H. 1984. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 105: 399–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

