DNA polymerase beta is critical for mouse meiotic synapsis
- PMID: 20019666
- PMCID: PMC2824467
- DOI: 10.1038/emboj.2009.357
DNA polymerase beta is critical for mouse meiotic synapsis
Abstract
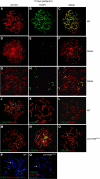
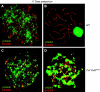
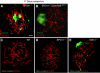
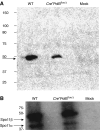
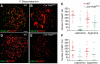
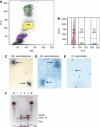
We have shown earlier that DNA polymerase beta (Pol beta) localizes to the synaptonemal complex (SC) during Prophase I of meiosis in mice. Pol beta localizes to synapsed axes during zygonema and pachynema, and it associates with the ends of bivalents during late pachynema and diplonema. To test whether these localization patterns reflect a function for Pol beta in recombination and/or synapsis, we used conditional gene targeting to delete the PolB gene from germ cells. We find that Pol beta-deficient spermatocytes are defective in meiotic chromosome synapsis and undergo apoptosis during Prophase I. We also find that SPO11-dependent gammaH2AX persists on meiotic chromatin, indicating that Pol beta is critical for the repair of SPO11-induced double-strand breaks (DSBs). Pol beta-deficient spermatocytes yielded reduced steady-state levels of the SPO11-oligonucleotide complexes that are formed when SPO11 is removed from the ends of DSBs, and cytological experiments revealed that chromosome-associated foci of replication protein A (RPA), RAD51 and DMC1 are less abundant in Pol beta-deficient spermatocyte nuclei. Localization of Pol beta to meiotic chromosomes requires the formation of SPO11-dependent DSBs. Taken together, these findings strongly indicate that Pol beta is required at a very early step in the processing of meiotic DSBs, at or before the removal of SPO11 from DSB ends and the generation of the 3' single-stranded tails necessary for subsequent strand exchange. The chromosome synapsis defects and Prophase I apoptosis of Pol beta-deficient spermatocytes are likely a direct consequence of these recombination defects.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
A surge of late-occurring meiotic double-strand breaks rescues synapsis abnormalities in spermatocytes of mice with hypomorphic expression of SPO11.Chromosoma. 2016 Jun;125(2):189-203. doi: 10.1007/s00412-015-0544-7. Epub 2015 Oct 6. Chromosoma. 2016. PMID: 26440409 Free PMC article.
-
The mouse Spo11 gene is required for meiotic chromosome synapsis.Mol Cell. 2000 Nov;6(5):975-87. doi: 10.1016/s1097-2765(00)00097-6. Mol Cell. 2000. PMID: 11106738
-
SPO11-independent DNA repair foci and their role in meiotic silencing.PLoS Genet. 2013 Jun;9(6):e1003538. doi: 10.1371/journal.pgen.1003538. Epub 2013 Jun 6. PLoS Genet. 2013. PMID: 23754961 Free PMC article.
-
DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis.Epigenetics. 2010 May 16;5(4):255-66. doi: 10.4161/epi.5.4.11518. Epub 2010 May 16. Epigenetics. 2010. PMID: 20364103 Review.
-
Cellular roles of DNA polymerase beta.Yale J Biol Med. 2013 Dec 13;86(4):463-9. Yale J Biol Med. 2013. PMID: 24348210 Free PMC article. Review.
Cited by
-
Targeting DNA polymerase ß for therapeutic intervention.Curr Mol Pharmacol. 2012 Jan;5(1):68-87. Curr Mol Pharmacol. 2012. PMID: 22122465 Free PMC article. Review.
-
Impairment of Pol β-related DNA base-excision repair leads to ovarian aging in mice.Aging (Albany NY). 2020 Nov 20;12(24):25207-25228. doi: 10.18632/aging.104123. Epub 2020 Nov 20. Aging (Albany NY). 2020. PMID: 33223510 Free PMC article.
-
Importance of genetic background for risk of relapse shown in altered prefrontal cortex gene expression during abstinence following chronic alcohol intoxication.Neuroscience. 2011 Jan 26;173:57-75. doi: 10.1016/j.neuroscience.2010.11.006. Epub 2010 Nov 25. Neuroscience. 2011. PMID: 21081154 Free PMC article.
-
A triad interaction in the fingers subdomain of DNA polymerase beta controls polymerase activity.J Am Chem Soc. 2011 Apr 27;133(16):6279-87. doi: 10.1021/ja111099b. Epub 2011 Mar 31. J Am Chem Soc. 2011. PMID: 21452873 Free PMC article.
-
DNA polymerases and cancer.Nat Rev Cancer. 2011 Feb;11(2):96-110. doi: 10.1038/nrc2998. Nat Rev Cancer. 2011. PMID: 21258395 Free PMC article. Review.
References
-
- Alcivar AA, Hake LE, Hecht NB (1992) DNA polymerase-beta and poly(ADP)ribose polymerase mRNAs are differentially expressed during the development of male germinal cells. Biol Reprod 46: 201–207 - PubMed
-
- Ashley T (2004) The mouse ‘tool box' for meiotic studies. Cytogenet Genome Res 105: 166–171 - PubMed
-
- Ashley T, Plug A (1998) Caught in the act: deducing meiotic function from protein immunolocalization. Curr Top Dev Biol 37: 201–239 - PubMed
-
- Ashley T, Plug AW, Xu J, Solari AJ, Reddy G, Golub EI, Ward DC (1995) Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma 104: 19–28 - PubMed
-
- Bannister LA, Schimenti JC (2004) Homologous recombinational repair proteins in mouse meiosis. Cytogenet Genome Res 107: 191–200 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

