Use of zebrafish to probe the divergent virulence potentials and toxin requirements of extraintestinal pathogenic Escherichia coli
- PMID: 20019794
- PMCID: PMC2785880
- DOI: 10.1371/journal.ppat.1000697
Use of zebrafish to probe the divergent virulence potentials and toxin requirements of extraintestinal pathogenic Escherichia coli
Abstract
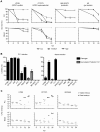
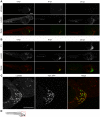
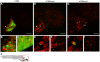
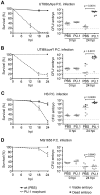
Extraintestinal pathogenic E. coli (ExPEC) cause an array of diseases, including sepsis, neonatal meningitis, and urinary tract infections. Many putative virulence factors that might modulate ExPEC pathogenesis have been identified through sequencing efforts, epidemiology, and gene expression profiling, but few of these genes have been assigned clearly defined functional roles during infection. Using zebrafish embryos as surrogate hosts, we have developed a model system with the ability to resolve diverse virulence phenotypes and niche-specific restrictions among closely related ExPEC isolates during either localized or systemic infections. In side-by-side comparisons of prototypic ExPEC isolates, we observed an unexpectedly high degree of phenotypic diversity that is not readily apparent using more traditional animal hosts. In particular, the capacity of different ExPEC isolates to persist and multiply within the zebrafish host and cause disease was shown to be variably dependent upon two secreted toxins, alpha-hemolysin and cytotoxic necrotizing factor. Both of these toxins appear to function primarily in the neutralization of phagocytes, which are recruited in high numbers to sites of infection where they act as an essential host defense against ExPEC as well as less virulent E. coli strains. These results establish zebrafish as a valuable tool for the elucidation and functional analysis of both ExPEC virulence factors and host defense mechanisms.
Conflict of interest statement
The authors have declared that no competing interests exist.
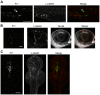
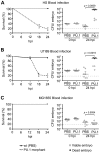
Figures



Similar articles
-
Comparative Genomics of Escherichia coli Isolated from Skin and Soft Tissue and Other Extraintestinal Infections.mBio. 2017 Aug 15;8(4):e01070-17. doi: 10.1128/mBio.01070-17. mBio. 2017. PMID: 28811343 Free PMC article.
-
Escherichia coli isolates from commercial chicken meat and eggs cause sepsis, meningitis and urinary tract infection in rodent models of human infections.Zoonoses Public Health. 2018 Feb;65(1):103-113. doi: 10.1111/zph.12376. Epub 2017 Jul 13. Zoonoses Public Health. 2018. PMID: 28703468
-
Combining quantitative genetic footprinting and trait enrichment analysis to identify fitness determinants of a bacterial pathogen.PLoS Genet. 2013;9(8):e1003716. doi: 10.1371/journal.pgen.1003716. Epub 2013 Aug 22. PLoS Genet. 2013. PMID: 23990803 Free PMC article.
-
Extraintestinal pathogenic Escherichia coli.Foodborne Pathog Dis. 2007 Summer;4(2):134-63. doi: 10.1089/fpd.2007.0087. Foodborne Pathog Dis. 2007. PMID: 17600482 Review.
-
What defines extraintestinal pathogenic Escherichia coli?Int J Med Microbiol. 2011 Dec;301(8):642-7. doi: 10.1016/j.ijmm.2011.09.006. Epub 2011 Oct 5. Int J Med Microbiol. 2011. PMID: 21982038 Review.
Cited by
-
Dissemination and systemic colonization of uropathogenic Escherichia coli in a murine model of bacteremia.mBio. 2010 Nov 23;1(5):e00262-10. doi: 10.1128/mBio.00262-10. mBio. 2010. PMID: 21116344 Free PMC article.
-
Context-Dependent Requirements for FimH and Other Canonical Virulence Factors in Gut Colonization by Extraintestinal Pathogenic Escherichia coli.Infect Immun. 2018 Feb 20;86(3):e00746-17. doi: 10.1128/IAI.00746-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29311232 Free PMC article.
-
The repeat-in-toxin family member TosA mediates adherence of uropathogenic Escherichia coli and survival during bacteremia.Infect Immun. 2012 Feb;80(2):493-505. doi: 10.1128/IAI.05713-11. Epub 2011 Nov 14. Infect Immun. 2012. PMID: 22083710 Free PMC article.
-
The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli.Infect Immun. 2013 May;81(5):1450-9. doi: 10.1128/IAI.01213-12. Epub 2013 Feb 19. Infect Immun. 2013. PMID: 23429541 Free PMC article.
-
Chromosomal complementation using Tn7 transposon vectors in Enterobacteriaceae.Appl Environ Microbiol. 2012 Sep;78(17):6001-8. doi: 10.1128/AEM.00986-12. Epub 2012 Jun 15. Appl Environ Microbiol. 2012. PMID: 22706059 Free PMC article.
References
-
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. - PubMed
-
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. - PubMed
-
- Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181:1753–1754. - PubMed
-
- Smith JL, Fratamico PM, Gunther NW. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog Dis. 2007;4:134–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

