Coordinated regulation of SIV replication and immune responses in the CNS
- PMID: 20019816
- PMCID: PMC2790080
- DOI: 10.1371/journal.pone.0008129
Coordinated regulation of SIV replication and immune responses in the CNS
Abstract
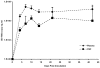
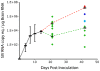
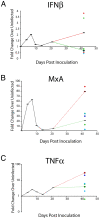
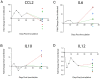
Central nervous system (CNS) invasion during acute-stage HIV-infection has been demonstrated in a small number of individuals, but there is no evidence of neurological impairment at this stage and virus infection in brain appears to be controlled until late-stage disease. Using our reproducible SIV macaque model to examine the earliest stages of infection in the CNS, we identified immune responses that differentially regulate inflammation and virus replication in the brain compared to the peripheral blood and lymphoid tissues. SIV replication in brain macrophages and in brain of SIV-infected macaques was detected at 4 days post-inoculation (p.i.). This was accompanied by upregulation of innate immune responses, including IFNbeta, IFNbeta-induced gene MxA mRNA, and TNFalpha. Additionally, IL-10, the chemokine CCL2, and activation markers in macrophages, endothelial cells, and astrocytes were all increased in the brain at four days p.i. We observed synchronous control of virus replication, cytokine mRNA levels and inflammatory markers (MHC Class II, CD68 and GFAP) by 14 days p.i.; however, control failure was followed by development of CNS lesions in the brain. SIV infection was accompanied by induction of the dominant-negative isoform of C/EBPbeta, which regulates SIV, CCL2, and IL6 transcription, as well as inflammatory responses in macrophages and astrocytes. This synchronous response in the CNS is in part due to the effect of the C/EBPbeta on virus replication and cytokine expression in macrophage-lineage cells in contrast to CD4+ lymphocytes in peripheral blood and lymphoid tissues. Thus, we have identified a crucial period in the brain when virus replication and inflammation are controlled. As in HIV-infected individuals, though, this control is not sustained in the brain. Our results suggest that intervention with antiretroviral drugs or anti-inflammatory therapeutics with CNS penetration would sustain early control. These studies further suggest that interventions should target HIV-infected individuals with increased CCL2 levels or HIV RNA in the CNS.
Conflict of interest statement
Figures


Similar articles
-
Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system.J Neurovirol. 2004;10 Suppl 1:15-20. doi: 10.1080/753312747. J Neurovirol. 2004. PMID: 14982734
-
Neuroprotective and anti-human immunodeficiency virus activity of minocycline.JAMA. 2005 Apr 27;293(16):2003-11. doi: 10.1001/jama.293.16.2003. JAMA. 2005. PMID: 15855434
-
Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir.mBio. 2017 Aug 15;8(4):e01186-17. doi: 10.1128/mBio.01186-17. mBio. 2017. PMID: 28811349 Free PMC article.
-
Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies.J Neurovirol. 2008 Aug;14(4):318-26. doi: 10.1080/13550280802132857. J Neurovirol. 2008. PMID: 18780233 Free PMC article. Review.
-
An SIV macaque model of SIV and HAND: the need for adjunctive therapies in HIV that target activated monocytes and macrophages.J Neurovirol. 2018 Apr;24(2):213-219. doi: 10.1007/s13365-018-0616-6. Epub 2018 Feb 12. J Neurovirol. 2018. PMID: 29435829 Review.
Cited by
-
PrPC, the cellular isoform of the human prion protein, is a novel biomarker of HIV-associated neurocognitive impairment and mediates neuroinflammation.Am J Pathol. 2010 Oct;177(4):1848-60. doi: 10.2353/ajpath.2010.091006. Epub 2010 Aug 19. Am J Pathol. 2010. PMID: 20724601 Free PMC article.
-
A plasma microRNA signature of acute lentiviral infection: biomarkers of central nervous system disease.AIDS. 2011 Nov 13;25(17):2057-67. doi: 10.1097/QAD.0b013e32834b95bf. AIDS. 2011. PMID: 21857495 Free PMC article.
-
HIV and neurocognitive dysfunction.Curr HIV/AIDS Rep. 2013 Sep;10(3):235-43. doi: 10.1007/s11904-013-0171-y. Curr HIV/AIDS Rep. 2013. PMID: 23860944 Free PMC article. Review.
-
Anti-inflammatory Function of Phyllostachys Edulis Extract in the Hippocampus of HIV-1 Transgenic Rats.J HIV AIDS. 2016 May;2(3):10.16966/2380-5536.126. doi: 10.16966/2380-5536.126. Epub 2016 May 11. J HIV AIDS. 2016. PMID: 27398410 Free PMC article.
-
Brain macrophages harbor latent, infectious simian immunodeficiency virus.AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
References
-
- Sinclair E, Gray F, Scaravilli F. PCR detection of HIV proviral DNA in the brain of an asymptomatic HIV-positive patient. J Neurol. 1992;239:469–470. - PubMed
-
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. - PubMed
-
- An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. - PubMed
-
- Gartner S. HIV infection and dementia. Science. 2000;287:602–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

