Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts
- PMID: 20019836
- PMCID: PMC2794509
- DOI: 10.1593/neo.09620
Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts
Abstract
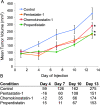
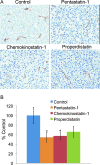
Angiogenesis or neovascularization, the process of new blood vessel formation from preexisting microvasculature, involves interactions among several cell types including parenchymal, endothelial cells, and immune cells. The formation of new vessels is tightly regulated by a balance between endogenous proangiogenic and antiangiogenic factors to maintain homeostasis in tissue; tumor progression and metastasis in breast cancer have been shown to be angiogenesis-dependent. We previously introduced a systematic methodology to identify putative endogenous antiangiogenic peptides and validated these predictions in vitro in human umbilical vein endothelial cell proliferation and migration assays. These peptides are derived from several protein families including type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins. On the basis of the results from the in vitro screening, we have evaluated the ability of one peptide selected from each family named pentastatin-1, chemokinostatin-1, and properdistatin, respectively, to suppress angiogenesis in an MDA-MB-231 human breast cancer orthotopic xenograft model in severe combined immunodeficient mice. Peptides were administered intraperitoneally once per day. We have demonstrated significant suppression of tumor growth in vivo and subsequent reductions in microvascular density, indicating the potential of these peptides as therapeutic agents for breast cancer.
Figures



Similar articles
-
Development of a biomimetic peptide derived from collagen IV with anti-angiogenic activity in breast cancer.Cancer Biol Ther. 2011 Nov 1;12(9):808-17. doi: 10.4161/cbt.12.9.17677. Epub 2011 Nov 1. Cancer Biol Ther. 2011. PMID: 21878750 Free PMC article.
-
Collagen IV and CXC chemokine-derived antiangiogenic peptides suppress glioma xenograft growth.Anticancer Drugs. 2012 Aug;23(7):706-12. doi: 10.1097/CAD.0b013e3283531041. Anticancer Drugs. 2012. PMID: 22495619 Free PMC article.
-
Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model.BMC Cancer. 2010 Feb 1;10:29. doi: 10.1186/1471-2407-10-29. BMC Cancer. 2010. PMID: 20122172 Free PMC article.
-
Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth.Prog Biophys Mol Biol. 2013 Nov;113(2):333-54. doi: 10.1016/j.pbiomolbio.2013.10.001. Epub 2013 Oct 15. Prog Biophys Mol Biol. 2013. PMID: 24139944 Review.
-
CXC chemokines in cancer angiogenesis and metastases.Adv Cancer Res. 2010;106:91-111. doi: 10.1016/S0065-230X(10)06003-3. Adv Cancer Res. 2010. PMID: 20399957 Free PMC article. Review.
Cited by
-
Thrombospondin-1 as a Paradigm for the Development of Antiangiogenic Agents Endowed with Multiple Mechanisms of Action.Pharmaceuticals (Basel). 2010 Apr 23;3(4):1241-1278. doi: 10.3390/ph3041241. Pharmaceuticals (Basel). 2010. PMID: 27713299 Free PMC article. Review.
-
AntiAngioPred: A Server for Prediction of Anti-Angiogenic Peptides.PLoS One. 2015 Sep 3;10(9):e0136990. doi: 10.1371/journal.pone.0136990. eCollection 2015. PLoS One. 2015. PMID: 26335203 Free PMC article.
-
Properdistatin inhibits angiogenesis and improves vascular function in human melanoma xenografts with low thrombospondin-1 expression.Oncotarget. 2016 Nov 22;7(47):76806-76815. doi: 10.18632/oncotarget.12695. Oncotarget. 2016. PMID: 27756886 Free PMC article.
-
Serpin-derived peptides are antiangiogenic and suppress breast tumor xenograft growth.Transl Oncol. 2012 Apr;5(2):92-7. doi: 10.1593/tlo.11244. Epub 2012 Apr 1. Transl Oncol. 2012. PMID: 22496925 Free PMC article.
-
Development of a biomimetic peptide derived from collagen IV with anti-angiogenic activity in breast cancer.Cancer Biol Ther. 2011 Nov 1;12(9):808-17. doi: 10.4161/cbt.12.9.17677. Epub 2011 Nov 1. Cancer Biol Ther. 2011. PMID: 21878750 Free PMC article.
References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
-
- Fernando NT, Koch M, Rothrock C, Gollogly LK, D'Amore PA, Ryeom S, Yoon SS. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin Cancer Res. 2008;14:1529–1539. - PubMed
-
- Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
