Retinal phototoxicity in a novel murine model of intraocular lens implantation
- PMID: 20019883
- PMCID: PMC2793903
Retinal phototoxicity in a novel murine model of intraocular lens implantation
Abstract
Purpose: To establish a novel murine intraocular lens (IOL) implantation model to study the protective effects of colored-IOLs against retinal phototoxicity.
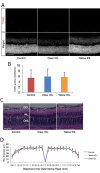
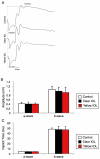
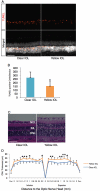
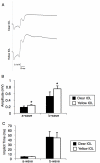
Methods: Two-millimeter diameter IOL buttons were created from IOLs for clinical use. Extra-capsular crystalline lens extraction and IOL implantation were performed in BALB/c mice using a technique similar to human cataract surgery. For light exposure experiments, mice were exposed to 5,000 LUX of white light for 24 h on the day after surgery. To investigate the protective effects of yellow IOL against light exposure, ERG measurements were conducted in vivo, followed by TdT-mediated dUTP Nick-End Labeling (TUNEL) and outer nuclear layer (ONL) thickness measurement of retinal tissue in yellow or clear IOL-implanted mice and control mice without surgery.
Results: IOLs were successfully implanted in all animals, and IOL buttons without haptics were well stabilized in the capsular bag. Murine eyes developed posterior capsule opacification (PCO) after IOL implantation by postoperative day 5 at the latest. In contrast to the clear IOL-implanted animals stimulated by light exposure, the yellow IOL-implanted animals had significantly reduced numbers of TUNEL-positive cells and retained thickness of the ONL. The ERG showed that yellow IOL implantation prevents a decrease of amplitude in both the a-wave and b-wave compared with clear IOL implantation.
Conclusions: We established a new animal model of IOL implantation and demonstrated the protective effects of colored-IOL against retinal phototoxicity after cataract surgery.
Figures


References
-
- Clark DS. Posterior capsule opacification. Curr Opin Ophthalmol. 2000;11:56–64. - PubMed
-
- Ray S, D'Amico DJ. Pseudophakic cystoid macular edema. Semin Ophthalmol. 2002;17:167–80. - PubMed
-
- Bockelbrink A, Roll S, Ruether K, Rasch A, Greiner W, Willich SN. Cataract surgery and the development or progression of age-related macular degeneration: a systematic review. Surv Ophthalmol. 2008;53:359–67. - PubMed
-
- Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, O'Colmain B, Wu SY, Taylor HR, Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–94. - PubMed
-
- Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
