How Ebola impacts genetics of Western lowland gorilla populations
- PMID: 20020045
- PMCID: PMC2791222
- DOI: 10.1371/journal.pone.0008375
How Ebola impacts genetics of Western lowland gorilla populations
Abstract
Background: Emerging infectious diseases in wildlife are major threats for both human health and biodiversity conservation. Infectious diseases can have serious consequences for the genetic diversity of populations, which could enhance the species' extinction probability. The Ebola epizootic in western and central Africa induced more than 90% mortality in Western lowland gorilla population. Although mortality rates are very high, the impacts of Ebola on genetic diversity of Western lowland gorilla have never been assessed.
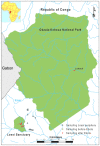
Methodology/principal findings: We carried out long term studies of three populations of Western lowland gorilla in the Republic of the Congo (Odzala-Kokoua National Park, Lossi gorilla sanctuary both affected by Ebola and Lossi's periphery not affected). Using 17 microsatellite loci, we compared genetic diversity and structure of the populations and estimate their effective size before and after Ebola outbreaks. Despite the effective size decline in both populations, we did not detect loss in genetic diversity after the epizootic. We revealed temporal changes in allele frequencies in the smallest population.
Conclusions/significance: Immigration and short time elapsed since outbreaks could explain the conservation of genetic diversity after the demographic crash. Temporal changes in allele frequencies could not be explained by genetic drift or random sampling. Immigration from genetically differentiated populations and a non random mortality induced by Ebola, i.e., selective pressure and cost of sociality, are alternative hypotheses. Understanding the influence of Ebola on gorilla genetic dynamics is of paramount importance for human health, primate evolution and conservation biology.
Conflict of interest statement
Figures

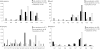
Similar articles
-
The genetic impact of an Ebola outbreak on a wild gorilla population.BMC Genomics. 2021 Oct 11;22(1):735. doi: 10.1186/s12864-021-08025-y. BMC Genomics. 2021. PMID: 34635054 Free PMC article.
-
Recovery potential of a western lowland gorilla population following a major Ebola outbreak: results from a ten year study.PLoS One. 2012;7(5):e37106. doi: 10.1371/journal.pone.0037106. Epub 2012 May 23. PLoS One. 2012. PMID: 22649511 Free PMC article.
-
Using demographic characteristics of populations to detect spatial fragmentation following suspected ebola outbreaks in great apes.Am J Phys Anthropol. 2017 Sep;164(1):3-10. doi: 10.1002/ajpa.23275. Epub 2017 Jun 29. Am J Phys Anthropol. 2017. PMID: 28661006
-
Ebola virus circulation in Africa: a balance between clinical expression and epidemiological silence.Bull Soc Pathol Exot. 2005 Sep;98(3):210-7. Bull Soc Pathol Exot. 2005. PMID: 16267963 Review.
-
Emerging infectious disease and the challenges of social distancing in human and non-human animals.Proc Biol Sci. 2020 Aug 12;287(1932):20201039. doi: 10.1098/rspb.2020.1039. Epub 2020 Aug 12. Proc Biol Sci. 2020. PMID: 32781952 Free PMC article. Review.
Cited by
-
Preclinical Development of Inactivated Rabies Virus-Based Polyvalent Vaccine Against Rabies and Filoviruses.J Infect Dis. 2015 Oct 1;212 Suppl 2(Suppl 2):S414-24. doi: 10.1093/infdis/jiv251. Epub 2015 Jun 10. J Infect Dis. 2015. PMID: 26063224 Free PMC article.
-
A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence.Virology. 2012 Dec 5;434(1):18-26. doi: 10.1016/j.virol.2012.07.020. Epub 2012 Aug 11. Virology. 2012. PMID: 22889613 Free PMC article.
-
The genetic impact of an Ebola outbreak on a wild gorilla population.BMC Genomics. 2021 Oct 11;22(1):735. doi: 10.1186/s12864-021-08025-y. BMC Genomics. 2021. PMID: 34635054 Free PMC article.
-
Discovery of gorilla MHC-C expressing C1 ligand for KIR.Immunogenetics. 2018 May;70(5):293-304. doi: 10.1007/s00251-017-1038-y. Epub 2017 Nov 3. Immunogenetics. 2018. PMID: 29101448 Free PMC article.
-
Mountain gorilla genomes reveal the impact of long-term population decline and inbreeding.Science. 2015 Apr 10;348(6231):242-245. doi: 10.1126/science.aaa3952. Epub 2015 Apr 9. Science. 2015. PMID: 25859046 Free PMC article.
References
-
- Altizer S, Harvell D, Friedle E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol Evol. 2003;18:589–596.
-
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife - Threats to biodiversity and human health. Science. 2000;287:443–449. - PubMed
-
- Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78:103–116. - PubMed
-
- Lafferty KD, Gerber L. Good medicine for conservation biology: The intersection of epidemiology and conservation theory. Conserv Biol. 2002;16:593–604.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

