Impaired terminal differentiation of hippocampal granule neurons and defective contextual memory in PC3/Tis21 knockout mice
- PMID: 20020054
- PMCID: PMC2791842
- DOI: 10.1371/journal.pone.0008339
Impaired terminal differentiation of hippocampal granule neurons and defective contextual memory in PC3/Tis21 knockout mice
Abstract
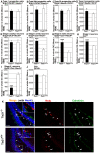
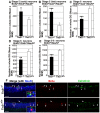
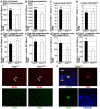
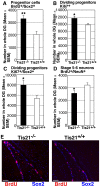
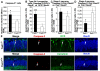
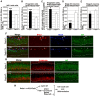
Neurogenesis in the dentate gyrus of the adult hippocampus has been implicated in neural plasticity and memory, but the molecular mechanisms controlling the proliferation and differentiation of newborn neurons and their integration into the synaptic circuitry are still largely unknown. To investigate this issue, we have analyzed the adult hippocampal neurogenesis in a PC3/Tis21-null mouse model. PC3/Tis21 is a transcriptional co-factor endowed with antiproliferative and prodifferentiative properties; indeed, its upregulation in neural progenitors has been shown to induce exit from cell cycle and differentiation. We demonstrate here that the deletion of PC3/Tis21 causes an increased proliferation of progenitor cells in the adult dentate gyrus and an arrest of their terminal differentiation. In fact, in the PC3/Tis21-null hippocampus postmitotic undifferentiated neurons accumulated, while the number of terminally differentiated neurons decreased of 40%. As a result, PC3/Tis21-null mice displayed a deficit of contextual memory. Notably, we observed that PC3/Tis21 can associate to the promoter of Id3, an inhibitor of proneural gene activity, and negatively regulates its expression, indicating that PC3/Tis21 acts upstream of Id3. Our results identify PC3/Tis21 as a gene required in the control of proliferation and terminal differentiation of newborn neurons during adult hippocampal neurogenesis and suggest its involvement in the formation of contextual memories.
Conflict of interest statement
Figures


Similar articles
-
Control of the Normal and Pathological Development of Neural Stem and Progenitor Cells by the PC3/Tis21/Btg2 and Btg1 Genes.J Cell Physiol. 2015 Dec;230(12):2881-90. doi: 10.1002/jcp.25038. J Cell Physiol. 2015. PMID: 25967096 Review.
-
Tis21 expression marks not only populations of neurogenic precursor cells but also new postmitotic neurons in adult hippocampal neurogenesis.Cereb Cortex. 2010 Feb;20(2):304-14. doi: 10.1093/cercor/bhp100. Epub 2009 May 29. Cereb Cortex. 2010. PMID: 19482889 Free PMC article.
-
The timing of differentiation of adult hippocampal neurons is crucial for spatial memory.PLoS Biol. 2008 Oct 7;6(10):e246. doi: 10.1371/journal.pbio.0060246. PLoS Biol. 2008. PMID: 18842068 Free PMC article.
-
BTG2(TIS21/PC3) induces neuronal differentiation and prevents apoptosis of terminally differentiated PC12 cells.Oncogene. 2002 Oct 3;21(44):6772-78. doi: 10.1038/sj.onc.1205888. Oncogene. 2002. PMID: 12360398
-
The gene PC3(TIS21/BTG2), prototype member of the PC3/BTG/TOB family: regulator in control of cell growth, differentiation, and DNA repair?J Cell Physiol. 2001 May;187(2):155-65. doi: 10.1002/jcp.1062. J Cell Physiol. 2001. PMID: 11267995 Review.
Cited by
-
miR-302 Is Required for Timing of Neural Differentiation, Neural Tube Closure, and Embryonic Viability.Cell Rep. 2015 Aug 4;12(5):760-73. doi: 10.1016/j.celrep.2015.06.074. Epub 2015 Jul 23. Cell Rep. 2015. PMID: 26212322 Free PMC article.
-
Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis.Endocrinology. 2012 Feb;153(2):759-69. doi: 10.1210/en.2011-1699. Epub 2011 Dec 6. Endocrinology. 2012. PMID: 22147012 Free PMC article.
-
Transcription-Factor-Dependent Control of Adult Hippocampal Neurogenesis.Cold Spring Harb Perspect Biol. 2015 Oct 1;7(10):a018879. doi: 10.1101/cshperspect.a018879. Cold Spring Harb Perspect Biol. 2015. PMID: 26430216 Free PMC article. Review.
-
Genome-wide transcriptomics of the amygdala reveals similar oligodendrocyte-related responses to acute and chronic alcohol drinking in female mice.Transl Psychiatry. 2022 Nov 12;12(1):476. doi: 10.1038/s41398-022-02231-2. Transl Psychiatry. 2022. PMID: 36371333 Free PMC article.
-
Hippocampal transcriptomic responses to enzyme-mediated cellular dissociation.Hippocampus. 2019 Sep;29(9):876-882. doi: 10.1002/hipo.23095. Epub 2019 May 14. Hippocampus. 2019. PMID: 31087609 Free PMC article.
References
-
- Tirone F. The gene PC3(TIS21/BTG2), prototype member of the PC3/BTG/TOB family: regulator in control of cell growth, differentiation, and DNA repair? J Cell Physiol. 2001;187:155–165. - PubMed
-
- Matsuda S, Rouault J, Magaud J, Berthet C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 2001;497:67–72. - PubMed
-
- Montagnoli A, Guardavaccaro D, Starace G, Tirone F. Overexpression of the nerve growth factor-inducible PC3 immediate early gene is associated with growth inhibition. Cell Growth Differ. 1996;7:1327–1336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

