Case reports: subtrochanteric femoral stress fractures after prolonged alendronate therapy
- PMID: 20020334
- PMCID: PMC2881993
- DOI: 10.1007/s11999-009-1192-0
Case reports: subtrochanteric femoral stress fractures after prolonged alendronate therapy
Abstract
Background: Alendronate is known for its ability to reduce bone loss in osteoporotic and osseous metastatic conditions. Its long-term effects remain unclear although several reports describe cases of proximal femur stress fractures associated with long-term alendronate use.
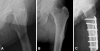
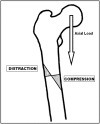
Case description: We report the cases of four women who sustained low-energy subtrochanteric or femoral shaft stress fractures while being on alendronate therapy for more than 5 years. All radiographs showed typical patterns consisting of a transverse fracture line with external cortical bone reaction and medial cortical spike. Alendronate discontinuation along with nonoperative treatment was sufficient for one patient, whereas surgical stabilization was required in three patients.
Literature review: The side effects of alendronate therapy include osteonecrosis of the jaw, esophageal irritation, and musculoskeletal pain. Several cases of insufficiency femoral fractures associated with prolonged alendronate use have been reported. Their radiographic pattern and clinical presentation are consistent with our observations. Although various hypotheses have been suggested, the physiopathogenesis of these stress fractures is not completely understood.
Purposes and clinical relevance: Although bisphosphonates play an important role in preventing pathologic fractures in patients with cancer, these subtrochanteric stress fractures associated with prolonged use of alendronate should not be ignored.
Figures



References
-
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/S0140-6736(96)07088-2. - DOI - PubMed
-
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

