Kinetic properties of ASC protein aggregation in epithelial cells
- PMID: 20020448
- PMCID: PMC10067259
- DOI: 10.1002/jcp.22005
Kinetic properties of ASC protein aggregation in epithelial cells
Abstract
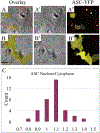
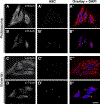
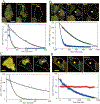
Apoptosis-associated speck-like protein with CARD domain (ASC), an adaptor protein composed of caspase recruitment and pyrin domains, can efficiently self-associate to form a large spherical structure, called a speck. Although ASC aggregation is generally involved with both inflammatory processes and apoptosis, the detailed dynamics of speck formation have not been characterized. In this report, speck formation in HeLa cells transfected with ASC is examined by time-lapse live-imaging by confocal laser scanning microscopy. The results show that ASC aggregation is a very rapid and tightly regulated process. Prior to speck formation, soluble ASC aggregation is a low probability event, and the affinity of ASC subunits for one another is very low. Following a speck nucleation event, the affinity for further addition of ASC subunits increases dramatically, and aggregation is a highly energetically favorable reaction (Gibbs free energy approximately -40 kJ/mol). This leads to a rapid depletion of soluble ASC, making it highly unlikely that a second speck will form inside the same cell and assuring that speck formation is "all or none," with a well-defined end point. Comparison with kinetic models of the aggregation process indicates diffusion, instead of active transport, is the dominant process for speck growth. Though speck formation and aggresome formation share some properties, we show that the two processes are distinct.
Figures




References
-
- Balci-Peynircioglu B, Waite AL, Schaner P, Taskiran ZE, Richards N, Orhan D, Gucer S, Ozen S, Gumucio D, Yilmaz E. 2008. Expression of ASC in Renal Tissues of Familial Mediterranean Fever Patients with Amyloidosis: Postulating a Role for ASC in AA Type Amyloid Deposition. Exp Biol Med (Maywood) 233(11):1324–1333. - PubMed
-
- Booth DR, Sunde M, Bellotti V, Robinson CV, Hutchinson WL, Fraser PE, Hawkins PN, Dobson CM, Radford SE, Blake CC, Pepys MB. 1997. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385(6619):787–793. - PubMed
-
- Carrell RW, Gooptu B. 1998. Conformational changes and disease--serpins, prions and Alzheimer’s. Curr Opin Struct Biol 8(6):799–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

