Does CTCF mediate between nuclear organization and gene expression?
- PMID: 20020479
- PMCID: PMC6375297
- DOI: 10.1002/bies.200900118
Does CTCF mediate between nuclear organization and gene expression?
Abstract
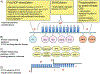
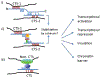
The multifunctional zinc-finger protein CCCTC-binding factor (CTCF) is a very strong candidate for the role of coordinating the expression level of coding sequences with their three-dimensional position in the nucleus, apparently responding to a "code" in the DNA itself. Dynamic interactions between chromatin fibers in the context of nuclear architecture have been implicated in various aspects of genome functions. However, the molecular basis of these interactions still remains elusive and is a subject of intense debate. Here we discuss the nature of CTCF-DNA interactions, the CTCF-binding specificity to its binding sites and the relationship between CTCF and chromatin, and we examine data linking CTCF with gene regulation in the three-dimensional nuclear space. We discuss why these features render CTCF a very strong candidate for the role and propose a unifying model, the "CTCF code," explaining the mechanistic basis of how the information encrypted in DNA may be interpreted by CTCF into diverse nuclear functions.
Figures

Similar articles
-
Genome-wide and parental allele-specific analysis of CTCF and cohesin DNA binding in mouse brain reveals a tissue-specific binding pattern and an association with imprinted differentially methylated regions.Genome Res. 2013 Oct;23(10):1624-35. doi: 10.1101/gr.150136.112. Epub 2013 Jun 26. Genome Res. 2013. PMID: 23804403 Free PMC article.
-
CTCF physically links cohesin to chromatin.Proc Natl Acad Sci U S A. 2008 Jun 17;105(24):8309-14. doi: 10.1073/pnas.0801273105. Epub 2008 Jun 11. Proc Natl Acad Sci U S A. 2008. PMID: 18550811 Free PMC article.
-
Cohesin is positioned in mammalian genomes by transcription, CTCF and Wapl.Nature. 2017 Apr 27;544(7651):503-507. doi: 10.1038/nature22063. Epub 2017 Apr 19. Nature. 2017. PMID: 28424523 Free PMC article.
-
Genome-wide studies of CCCTC-binding factor (CTCF) and cohesin provide insight into chromatin structure and regulation.J Biol Chem. 2012 Sep 7;287(37):30906-13. doi: 10.1074/jbc.R111.324962. Epub 2012 Sep 5. J Biol Chem. 2012. PMID: 22952237 Free PMC article. Review.
-
CTCF and cohesin: linking gene regulatory elements with their targets.Cell. 2013 Mar 14;152(6):1285-97. doi: 10.1016/j.cell.2013.02.029. Cell. 2013. PMID: 23498937 Review.
Cited by
-
A Newly Assigned Role of CTCF in Cellular Response to Broken DNAs.Biomolecules. 2021 Feb 27;11(3):363. doi: 10.3390/biom11030363. Biomolecules. 2021. PMID: 33673494 Free PMC article. Review.
-
WNT signaling and AHCTF1 promote oncogenic MYC expression through super-enhancer-mediated gene gating.Nat Genet. 2019 Dec;51(12):1723-1731. doi: 10.1038/s41588-019-0535-3. Epub 2019 Nov 29. Nat Genet. 2019. PMID: 31784729
-
CTCF and CTCFL mRNA expression in 17β-estradiol-treated MCF7 cells.Biomed Rep. 2014 Jan;2(1):101-104. doi: 10.3892/br.2013.200. Epub 2013 Nov 15. Biomed Rep. 2014. PMID: 24649078 Free PMC article.
-
Coordination of KSHV latent and lytic gene control by CTCF-cohesin mediated chromosome conformation.PLoS Pathog. 2011 Aug;7(8):e1002140. doi: 10.1371/journal.ppat.1002140. Epub 2011 Aug 18. PLoS Pathog. 2011. PMID: 21876668 Free PMC article.
-
Deciphering the DNA code for the function of the Drosophila polydactyl zinc finger protein Suppressor of Hairy-wing.Nucleic Acids Res. 2017 May 5;45(8):4463-4478. doi: 10.1093/nar/gkx040. Nucleic Acids Res. 2017. PMID: 28158673 Free PMC article.
References
-
- Goetze S, Mateos-Langerak J, van Driel R. 2007. Three-dimensional genome organization in interphase and its relation to genome function. Semin Cell Dev Biol 18: 707–14. - PubMed
-
- Cremer T, Cremer C. 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2: 292–301. - PubMed
-
- Sexton T, Schober H, Fraser P, et al. 2007. Gene regulation through nuclear organization. Nat Struct Mol Biol 14: 1049–1055. - PubMed
-
- Gilbert N, Gilchrist S, Bickmore WA. 2005. Chromatin organization in the mammalian nucleus. Int Rev Cytol 242: 283–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

