Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish
- PMID: 20020540
- PMCID: PMC2976675
- DOI: 10.1002/cne.22233
Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish
Abstract
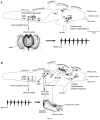
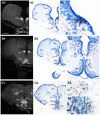
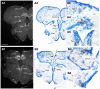
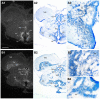
Across all major vertebrate groups, androgen receptors (ARs) have been identified in neural circuits that shape reproductive-related behaviors, including vocalization. The vocal control network of teleost fishes presents an archetypal example of how a vertebrate nervous system produces social, context-dependent sounds. We cloned a partial cDNA of AR that was used to generate specific probes to localize AR expression throughout the central nervous system of the vocal plainfin midshipman fish (Porichthys notatus). In the forebrain, AR mRNA is abundant in proposed homologs of the mammalian striatum and amygdala, and in anterior and posterior parvocellular and magnocellular nuclei of the preoptic area, nucleus preglomerulosus, and posterior, ventral and anterior tuberal nuclei of the hypothalamus. Many of these nuclei are part of the known vocal and auditory circuitry in midshipman. The midbrain periaqueductal gray, an essential link between forebrain and hindbrain vocal circuitry, and the lateral line recipient nucleus medialis in the rostral hindbrain also express abundant AR mRNA. In the caudal hindbrain-spinal vocal circuit, high AR mRNA is found in the vocal prepacemaker nucleus and along the dorsal periphery of the vocal motor nucleus congruent with the known pattern of expression of aromatase-containing glial cells. Additionally, abundant AR mRNA expression is shown for the first time in the inner ear of a vertebrate. The distribution of AR mRNA strongly supports the role of androgens as modulators of behaviorally defined vocal, auditory, and neuroendocrine circuits in teleost fish and vertebrates in general.
2009 Wiley-Liss, Inc.
Figures








Similar articles
-
Melatonin receptor expression in vocal, auditory, and neuroendocrine centers of a highly vocal fish, the plainfin midshipman (Porichthys notatus).J Comp Neurol. 2019 Jun 1;527(8):1362-1377. doi: 10.1002/cne.24629. Epub 2019 Jan 22. J Comp Neurol. 2019. PMID: 30620047
-
Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits.J Comp Neurol. 2002 Jul 1;448(3):298-322. doi: 10.1002/cne.10258. J Comp Neurol. 2002. PMID: 12115710
-
Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression.J Comp Neurol. 2005 Feb 28;483(1):91-113. doi: 10.1002/cne.20397. J Comp Neurol. 2005. PMID: 15672394
-
Neuroendocrine control of seasonal plasticity in the auditory and vocal systems of fish.Front Neuroendocrinol. 2015 Apr;37:129-45. doi: 10.1016/j.yfrne.2014.08.002. Epub 2014 Aug 26. Front Neuroendocrinol. 2015. PMID: 25168757 Free PMC article. Review.
-
Auditory signal processing in communication: perception and performance of vocal sounds.Hear Res. 2013 Nov;305:144-55. doi: 10.1016/j.heares.2013.06.007. Epub 2013 Jul 1. Hear Res. 2013. PMID: 23827717 Free PMC article. Review.
Cited by
-
A Review on Sex Steroid Hormone Estrogen Receptors in Mammals and Fish.Int J Endocrinol. 2020 Feb 7;2020:5386193. doi: 10.1155/2020/5386193. eCollection 2020. Int J Endocrinol. 2020. PMID: 32089683 Free PMC article. Review.
-
Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning.Curr Opin Neurobiol. 2010 Dec;20(6):748-53. doi: 10.1016/j.conb.2010.08.007. Epub 2010 Sep 9. Curr Opin Neurobiol. 2010. PMID: 20829032 Free PMC article. Review.
-
Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish.J Exp Biol. 2011 Jun 1;214(Pt 11):1931-42. doi: 10.1242/jeb.054114. J Exp Biol. 2011. PMID: 21562181 Free PMC article.
-
Sex differences in neuromuscular androgen receptor expression and sociosexual behavior in a sex changing fish.PLoS One. 2017 May 16;12(5):e0177711. doi: 10.1371/journal.pone.0177711. eCollection 2017. PLoS One. 2017. PMID: 28520775 Free PMC article.
-
Neural Mechanisms Underlying the Disruption of Male Courtship Behavior by Adult Exposure to Di(2-ethylhexyl) Phthalate in Mice.Environ Health Perspect. 2017 Sep 1;125(9):097001. doi: 10.1289/EHP1443. Environ Health Perspect. 2017. PMID: 28934723 Free PMC article.
References
-
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–178. - PubMed
-
- Bass AH. Shaping brain sexuality. Am Sci. 1996;84:352–363.
-
- Bass AH. Steroid-dependent plasticity of vocal motor systems: novel insights from teleost fish. Brain Res Rev. 2008;57:299–308. - PubMed
-
- Bass AH, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

