On the chalcogenophilicity of mercury: evidence for a strong Hg-Se bond in [Tm(Bu(t))]HgSePh and its relevance to the toxicity of mercury
- PMID: 20020759
- PMCID: PMC2810633
- DOI: 10.1021/ja907523x
On the chalcogenophilicity of mercury: evidence for a strong Hg-Se bond in [Tm(Bu(t))]HgSePh and its relevance to the toxicity of mercury
Abstract
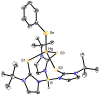
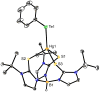
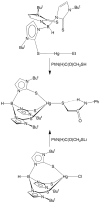
One of the reasons for the toxic effects of mercury has been attributed to its influence on the biochemical roles of selenium. For this reason, it is important to understand details pertaining to the nature of Hg-Se interactions and this has been achieved by comparison of a series of mercury chalcogenolate complexes that are supported by tris(2-mercapto-1-t-butyl-imidazolyl)hydroborato ligation, namely [Tm(Bu(t))]HgEPh (E = S, Se, Te). In particular, X-ray diffraction studies on [Tm(Bu(t))]HgEPh demonstrate that although the Hg-S bonds involving the [Tm(Bu(t))] ligand are longer than the corresponding Cd-S bonds of [Tm(Bu(t))]CdEPh, the Hg-EPh bonds are actually shorter than the corresponding Cd-EPh bonds, an observation which indicates that the apparent covalent radii of the metals in these compounds are dependent on the nature of the bonds. Furthermore, the difference in Hg-EPh and Cd-EPh bond lengths is a function of the chalcogen and increases in the sequence S (0.010 A) < Se (0.035 A) < Te (0.057 A). This trend indicates that the chalcogenophilicity of mercury increases in the sequence S < Se < Te. Thus, while mercury is often described as being thiophilic, it is evident that it actually has a greater selenophilicity, a notion that is supported by the observation of facile selenolate transfer from zinc to mercury upon treatment of [Tm(Bu(t))]HgSCH(2)C(O)N(H)Ph with [Tm(Bu(t))]ZnSePh. The significant selenophilicity of mercury is in accord with the aforementioned proposal that one reason for the toxicity of mercury is associated with it reducing the bioavailability of selenium.
Figures





Similar articles
-
Chalcogenophilicity of mercury.Inorg Chem. 2011 Apr 18;50(8):3791-8. doi: 10.1021/ic200199b. Epub 2011 Mar 15. Inorg Chem. 2011. PMID: 21405027
-
Methyl and arylchalcogenolate complexes of cadmium in a sulfur rich coordination environment: syntheses and structural characterization of the tris(2-mercapto-1-tert-butylimidazolyl)hydroborato cadmium complexes [Tm(Bu(t))]CdMe, and [Tm(Bu(t))]CdEAr (E = O, S, Se, Te) and analysis of the bonding in chalcogenolate compounds.Dalton Trans. 2006 Sep 21;(35):4207-10. doi: 10.1039/b607916d. Epub 2006 Jul 31. Dalton Trans. 2006. PMID: 16932812
-
Oxidative addition to U(V)-U(V) dimers: facile routes to uranium(VI) bis(imido) complexes.Inorg Chem. 2009 Dec 21;48(24):11615-23. doi: 10.1021/ic901581r. Inorg Chem. 2009. PMID: 19947591
-
Revision of reciprocal action of mercury and selenium.Int J Occup Med Environ Health. 2018 Oct 23;31(5):575-592. doi: 10.13075/ijomeh.1896.01278. Epub 2018 Jun 18. Int J Occup Med Environ Health. 2018. PMID: 29911664 Review.
-
Selenium as an antidote in the treatment of mercury intoxication.Biometals. 2015 Aug;28(4):605-14. doi: 10.1007/s10534-015-9857-5. Epub 2015 May 7. Biometals. 2015. PMID: 25947386 Review.
Cited by
-
Tris(2-mercaptoimidazolyl)hydroborato Cadmium Thiolate Complexes, [TmBut]CdSAr: Thiolate Exchange at Cadmium in a Sulfur-Rich Coordination Environment.Inorg Chem. 2017 Apr 17;56(8):4644-4654. doi: 10.1021/acs.inorgchem.7b00296. Epub 2017 Apr 3. Inorg Chem. 2017. PMID: 28368611 Free PMC article.
-
Synthesis and structures of cadmium carboxylate and thiocarboxylate compounds with a sulfur-rich coordination environment: carboxylate exchange kinetics involving tris(2-mercapto-1-t-butylimidazolyl)hydroborato cadmium complexes, [Tm(Bu(t))]Cd(O2CR).Inorg Chem. 2015 Apr 20;54(8):3835-50. doi: 10.1021/acs.inorgchem.5b00017. Epub 2015 Mar 31. Inorg Chem. 2015. PMID: 25826184 Free PMC article.
-
Structural characterization of 1,3-propanedithiols that feature carboxylic acids: Homologues of mercury chelating agents.Polyhedron. 2013 Nov 12;64:10.1016/j.poly.2013.05.012. doi: 10.1016/j.poly.2013.05.012. Polyhedron. 2013. PMID: 24187425 Free PMC article.
-
Synthesis and structural characterization of tris(2-mercapto-1-methylbenzimidazolyl)hydroborato cadmium halide complexes, {[Tm(MeBenz)]Cd(μ-Cl)}2 and [Tm(MeBenz)]CdI: a rare example of cadmium in a trigonal bipyramidal sulfur-rich coordination environment.Dalton Trans. 2014 Oct 7;43(37):13874-82. doi: 10.1039/c4dt01820f. Epub 2014 Aug 8. Dalton Trans. 2014. PMID: 25105778 Free PMC article.
-
First-in-Class Selenium-Containing Potent Serotonin Receptor 5-HT6 Agents with a Beneficial Neuroprotective Profile against Alzheimer's Disease.J Med Chem. 2024 Jan 25;67(2):1580-1610. doi: 10.1021/acs.jmedchem.3c02148. Epub 2024 Jan 8. J Med Chem. 2024. PMID: 38190615 Free PMC article.
References
-
- Clarkson TW, Magos L. Crit Rev Toxicol. 2006;36:609–662. - PubMed
- Mutter J, Naumann J, Guethlin C. Crit Rev Toxicol. 2007;37:537–549. - PubMed
- Clarkson TW. Env Health Persp Suppl. 2002;110:11–23. - PMC - PubMed
- Clarkson TW. Crit Rev Clin Lab Sci. 1997;34:369–403. - PubMed
- Langford NJ, Ferner RE. J Human Hypertension. 1999;13:651–656. - PubMed
- Boening DW. Chemosphere. 2000;40:1335–1351. - PubMed
- Magos L. Metal Ions in Biological Systems. 1997;34:321–370. - PubMed
- Hutchison AR, Atwood DA. J Chem Crystallogr. 2003;33:631–645.
- Alessio L, Campagna M, Lucchini R. Am J Ind Med. 2007;50:779–787. - PubMed
- Clarkson TW, Vyas JB, Ballatorl N. Am J Ind Med. 2007;50:757–764. - PubMed
- Risher JF, De Rosa CT. J Env Health. 2007;70:9–16. - PubMed
- Onyido I, Norris AR, Buncel E. Chem Rev. 2004;104:5911–5929. - PubMed
- Ozuah PO. Curr Probl Pediatr. 2000;30:91–99. - PubMed
-
- Tai HC, Lim C. J Phys Chem A. 2006;110:452–462. - PubMed
-
- Prince RC, Gailer J, Gunson DE, Turner RJ, George GN, Pickering IJ. J Inorg Biochem. 2007;101:1891–1893. - PubMed
- Gailer J. Coord Chem Rev. 2007;251:234–254.
- Gailer J. Appl Organometal Chem. 2002;16:701–707.
- Cuvin-Aralar MLA, Furness RW. Ecotoxicol Env Safety. 1991;21:348–364. - PubMed
- Yang DY, Chen YW, Gunn JM, Belzile N. Selenium and mercury in organisms: Interactions and mechanisms. Environ Rev. 2008;16:71–92.
- Ikemoto T, Kunito T, Tanaka H, Baba N, Miyazaki N, Tanabe S. Arch Environ Cont Toxicol. 2004;47:402–413. - PubMed
- Magos L, Webb M, Clarkson TW. Crit Rev Toxicol. 1980;8:1–42. - PubMed
- Soldin OP, O'Mara DM, Aschner M. Biol Trace Elem Res. 2008;126:1–12. - PMC - PubMed
- Whanger PD. J Trace Elem Electrolytes Health Dis. 1992;6:209–221. - PubMed
- Kaur P, Evje L, Aschner M, Syversen T. Toxicol Vitro. 2009;23:378–385. - PubMed
- Peterson SA, Ralston NVC, Peck DV, Van Sickle J, Robertson JD, Spate VL, Morris JS. Environ Sci Technol. 2009;43:3919–3925. - PubMed
- Seppänen K, Soininen P, Salonen JT, Lötjönen S, Laatikainen R. Biol Trace Elem Res. 2004;101:117–132. - PubMed
- Ralston NVC, Ralston CR, Blackwell JL, III, Raymond LJ. Neurotoxicology. 2008;29:802–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

