Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery
- PMID: 20020773
- PMCID: PMC2897173
- DOI: 10.1021/pr901076y
Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery
Abstract
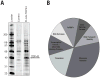
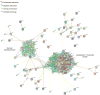
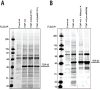
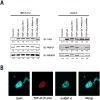
TDP-43 is a highly conserved and ubiquitously expressed member of the heterogeneous nuclear ribonucleoprotein (hnRNP) family of proteins. Recently, TDP-43 was shown to be a major disease protein in the ubiquitinated inclusions characteristic of most cases of amyotrophic lateral sclerosis (ALS), tau-negative frontotemporal lobar degeneration (FTLD), and inclusion body myopathy. In these diseases, TDP-43 is redistributed from its predominantly nuclear location to ubiquitin-positive, cytoplasmic foci. The extent to which TDP-43 drives pathophysiology is unknown, but the identification of mutations in TDP-43 in familial forms of ALS and FTLD-U suggests an important role for this protein in pathogenesis. Little is known about TDP-43 function and only a few TDP-43 interacting proteins have been previously identified, which makes further insight into both the normal and pathological functions of TDP-43 difficult. Here we show, via a global proteomic approach, that TDP-43 has extensive interaction with proteins that regulate RNA metabolism. Some interactions with TDP-43 were found to be dependent on RNA-binding, whereas other interactions are RNA-independent. Disease-causing mutations in TDP-43 (A315T and M337V) do not alter its interaction profile. TDP-43 interacting proteins largely cluster into two distinct interaction networks, a nuclear/splicing cluster and a cytoplasmic/translation cluster, strongly suggesting that TDP-43 has multiple roles in RNA metabolism and functions in both the nucleus and the cytoplasm. Finally, we found numerous TDP-43 interactors that are known components of stress granules, and indeed, we find that TDP-43 is also recruited to stress granules.
Figures

Similar articles
-
Increased cytoplasmic TDP-43 reduces global protein synthesis by interacting with RACK1 on polyribosomes.Hum Mol Genet. 2017 Apr 15;26(8):1407-1418. doi: 10.1093/hmg/ddx035. Hum Mol Genet. 2017. PMID: 28158562 Free PMC article.
-
Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue.PLoS One. 2010 Oct 11;5(10):e13250. doi: 10.1371/journal.pone.0013250. PLoS One. 2010. PMID: 20948999 Free PMC article.
-
Conjoint pathologic cascades mediated by ALS/FTLD-U linked RNA-binding proteins TDP-43 and FUS.Neurology. 2011 Oct 25;77(17):1636-43. doi: 10.1212/WNL.0b013e3182343365. Epub 2011 Sep 28. Neurology. 2011. PMID: 21956718 Free PMC article. Review.
-
TDP-43 mutations causing amyotrophic lateral sclerosis are associated with altered expression of RNA-binding protein hnRNP K and affect the Nrf2 antioxidant pathway.Hum Mol Genet. 2017 May 1;26(9):1732-1746. doi: 10.1093/hmg/ddx093. Hum Mol Genet. 2017. PMID: 28334913
-
Mechanisms Associated with TDP-43 Neurotoxicity in ALS/FTLD.Adv Neurobiol. 2018;20:239-263. doi: 10.1007/978-3-319-89689-2_9. Adv Neurobiol. 2018. PMID: 29916022 Review.
Cited by
-
Stress granules inhibit fatty acid oxidation by modulating mitochondrial permeability.Cell Rep. 2021 Jun 15;35(11):109237. doi: 10.1016/j.celrep.2021.109237. Cell Rep. 2021. PMID: 34133922 Free PMC article.
-
TDP-43 regulates β-adducin (Add2) transcript stability.RNA Biol. 2014;11(10):1280-90. doi: 10.1080/15476286.2014.996081. RNA Biol. 2014. PMID: 25602706 Free PMC article.
-
TDP-43 identified from a genome wide RNAi screen for SOD1 regulators.PLoS One. 2012;7(4):e35818. doi: 10.1371/journal.pone.0035818. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563406 Free PMC article.
-
TDP-43 and other hnRNPs regulate cryptic exon inclusion of a key ALS/FTD risk gene, UNC13A.PLoS Biol. 2023 Mar 17;21(3):e3002028. doi: 10.1371/journal.pbio.3002028. eCollection 2023 Mar. PLoS Biol. 2023. PMID: 36930682 Free PMC article.
-
A novel S379A TARDBP mutation associated to late-onset sporadic ALS.Neurol Sci. 2019 Oct;40(10):2111-2118. doi: 10.1007/s10072-019-03943-y. Epub 2019 Jun 4. Neurol Sci. 2019. PMID: 31165305
References
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–3. - PubMed
-
- Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66(2):152–7. - PubMed
-
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–72. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

