Temporal characteristics of botulinum neurotoxin therapy
- PMID: 20021324
- PMCID: PMC2842014
- DOI: 10.1586/ern.09.134
Temporal characteristics of botulinum neurotoxin therapy
Abstract
Botulinum neurotoxin is a pharmaceutical treatment used for an increasing number of neurological and non-neurological indications, symptoms and diseases. Despite the wealth of clinical reports that involve the timing of the therapeutic effects of this toxin, few studies have attempted to integrate these data into unified models. Secondary reactions have also been examined including the development of adverse events, resistance to repeated applications, and nerve terminal sprouting. Our primary intent for conducting this review was to gather relevant pharmacodynamic data from suitable biomedical literature regarding botulinum neurotoxins via the use of automated data-mining techniques. We envision that mathematical models will ultimately be of value to those who are healthcare decision makers and providers, as well as clinical and basic researchers. Furthermore, we hypothesize that the combination of this computer-intensive approach with mathematical modeling will predict the percentage of patients who will favorably or adversely respond to this treatment and thus will eventually assist in developing the increasingly important area of personalized medicine.
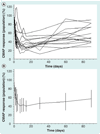
Figures
Similar articles
-
Botulinum toxin: a deadly substance with great therapeutic effect.Prof Nurse. 2003 Oct;19(2):85-7. Prof Nurse. 2003. PMID: 14593781 Review.
-
Botulinum toxin--mechanisms of action and clinical use in spasticity.J Rehabil Med. 2003 May;(41 Suppl):56-9. doi: 10.1080/16501960310010151. J Rehabil Med. 2003. PMID: 12817658 Review.
-
[Mechanism of action and therapeutic uses of botulinum and tetanus neurotoxins].Ann Pharm Fr. 2001 May;59(3):176-90. Ann Pharm Fr. 2001. PMID: 11427819 Review. French.
-
Xeomin: an innovative new botulinum toxin type A.Eur J Neurol. 2009 Dec;16 Suppl 2:11-3. doi: 10.1111/j.1468-1331.2009.02879.x. Eur J Neurol. 2009. PMID: 20002741 Review.
-
Botulinum and tetanus neurotoxins: structure, function and therapeutic utility.Trends Biochem Sci. 2002 Nov;27(11):552-8. doi: 10.1016/s0968-0004(02)02177-1. Trends Biochem Sci. 2002. PMID: 12417130 Review.
Cited by
-
IGF-1 antibody prolongs the effective duration time of botulinum toxin in decreasing muscle strength.Int J Mol Sci. 2013 Apr 25;14(5):9051-61. doi: 10.3390/ijms14059051. Int J Mol Sci. 2013. PMID: 23698763 Free PMC article.
-
Systems analysis of transcriptional data provides insights into muscle's biological response to botulinum toxin.Muscle Nerve. 2014 Nov;50(5):744-58. doi: 10.1002/mus.24211. Epub 2014 Mar 17. Muscle Nerve. 2014. PMID: 24536034 Free PMC article.
-
Botulinum Toxin-Chitosan Nanoparticles Prevent Arrhythmia in Experimental Rat Models.Mar Drugs. 2020 Aug 2;18(8):410. doi: 10.3390/md18080410. Mar Drugs. 2020. PMID: 32748868 Free PMC article.
-
The zinc-dependent protease activity of the botulinum neurotoxins.Toxins (Basel). 2010 May;2(5):978-97. doi: 10.3390/toxins2050978. Epub 2010 May 7. Toxins (Basel). 2010. PMID: 22069621 Free PMC article. Review.
-
Nano drugs delivery system: A novel promise for the treatment of atrial fibrillation.Front Cardiovasc Med. 2022 Oct 26;9:906350. doi: 10.3389/fcvm.2022.906350. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36386310 Free PMC article. Review.
References
-
- Adam OR, Jankovic J. Treatment of dystonia. Parkinsonism Relat. Disord. 2007;13 Suppl. 3:S362–S368. - PubMed
-
- Simpson LL. Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol. 2004;44:167–193. - PubMed
-
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. - PubMed
-
- Morbiato L, Carli L, Johnson EA, Montecucco C, Molgo J, Rossetto O. Neuromuscular paralysis and recovery in mice injected with botulinum neurotoxins A and C. Eur. J. Neurosci. 2007;25:2697–2704. - PubMed
-
- Lawrence G, Wang J, Chion CK, Aoki KR, Dolly JO. Two protein trafficking processes at motor nerve endings unveiled by botulinum neurotoxin E. J. Pharmacol. Exp. Ther. 2007;320:410–418. - PubMed
Websites
-
- Martin B. Botulinum toxin: the name game. [Accessed October 2009]. http://bmartinmd.com/2009/08/botulinum-toxin-the-name-game.html.
-
- BotDB database. http://botdb.abcc.ncifcrf.gov.
-
- Anonymous. FDA gives update on botulinum toxin safety warnings; established names of drugs changed. [Accessed October 2009]. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm175013.htm.
-
- Allergan. Botox® cosmetic (onabotulinumtoxinA) for injection. 2009. [Accessed October 2009]. www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
