Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties
- PMID: 20021667
- PMCID: PMC2807857
- DOI: 10.1186/1479-5876-7-109
Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties
Abstract
Background: Optimization of the current dendritic cell (DC) culture protocol in order to promote the therapeutic efficacy of DC-based immunotherapy is warranted. Alternative differentiation of monocyte-derived DCs using granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-15 has been propagated as an attractive strategy in that regard. The applicability of these so-called IL-15 DCs has not yet been firmly established. We therefore developed a novel pre-clinical approach for the generation of IL-15 DCs with potent immunostimulatory properties.
Methods: Human CD14+ monocytes were differentiated with GM-CSF and IL-15 into immature DCs. Monocyte-derived DCs, conventionally differentiated in the presence of GM-CSF and IL-4, served as control. Subsequent maturation of IL-15 DCs was induced using two clinical grade maturation protocols: (i) a classic combination of pro-inflammatory cytokines (tumor necrosis factor-alpha, IL-1beta, IL-6, prostaglandin E2) and (ii) a Toll-like receptor (TLR)7/8 agonist-based cocktail (R-848, interferon-gamma, TNF-alpha and prostaglandin E2). In addition, both short-term (2-3 days) and long-term (6-7 days) DC culture protocols were compared. The different DC populations were characterized with respect to their phenotypic profile, migratory properties, cytokine production and T cell stimulation capacity.
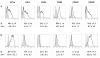
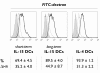
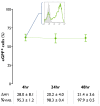
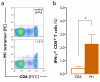
Results: The use of a TLR7/8 agonist-based cocktail resulted in a more optimal maturation of IL-15 DCs, as reflected by the higher phenotypic expression of CD83 and costimulatory molecules (CD70, CD80, CD86). The functional superiority of TLR7/8-activated IL-15 DCs over conventionally matured IL-15 DCs was evidenced by their (i) higher migratory potential, (ii) advantageous cytokine secretion profile (interferon-gamma, IL-12p70) and (iii) superior capacity to stimulate autologous, antigen-specific T cell responses after passive peptide pulsing. Aside from a less pronounced production of bioactive IL-12p70, short-term versus long-term culture of TLR7/8-activated IL-15 DCs resulted in a migratory profile and T cell stimulation capacity that was in favour of short-term DC culture. In addition, we demonstrate that mRNA electroporation serves as an efficient antigen loading strategy of IL-15 DCs.
Conclusions: Here we show that short-term cultured and TLR7/8-activated IL-15 DCs fulfill all pre-clinical prerequisites of immunostimulatory DCs. The results of the present study might pave the way for the implementation of IL-15 DCs in immunotherapy protocols.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

