Dry needling and exercise for chronic whiplash - a randomised controlled trial
- PMID: 20021675
- PMCID: PMC2805606
- DOI: 10.1186/1471-2474-10-160
Dry needling and exercise for chronic whiplash - a randomised controlled trial
Abstract
Background: Chronic whiplash is a common and costly problem. Sensory hypersensitivity is a feature of chronic whiplash that is associated with poor responsiveness to physical treatments such as exercise. Modalities such as dry-needling have shown some capacity to modulate sensory hypersensitivity, suggesting that when combined with advice and exercise, such an approach may be more effective in the management of chronic whiplash. The primary aim of this project is to investigate the effectiveness of dry-needling, advice and exercise for chronic whiplash.
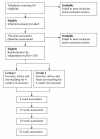
Method/design: A double-blind randomised controlled trial will be conducted. 120 participants with chronic whiplash, grade II will be randomised to receive either 1) dry-needling, advice and exercise or 2) sham dry-needling, advice and exercise. All participants will receive an educational booklet on whiplash. Participants who are randomised to Group 1 will receive 6 treatments of combined dry-needling and exercise delivered in the first 3 weeks of the 6 week program, and 4 treatments of exercise only in the last 3 weeks of the program. Participants randomised to Group 2 will receive an identical protocol, except that a sham dry-needling technique will be used instead of dry-needling. The primary outcome measures are the Neck Disability Index (NDI) and participants' perceived recovery. Outcomes will be measured at 6, 12, 24 and 52 weeks after randomization by an assessor who is blind to the group allocation of the participants. In parallel, an economic analysis will be conducted.
Discussion: This trial will utilise high quality trial methodologies in accordance with CONSORT guidelines. The successful completion of this trial will provide evidence of the effectiveness and cost-effectiveness of a combined treatment approach for the management of chronic whiplash.
Trial registration: ACTRN12609000470291.
References
-
- MAIC. Annual report 2003-4. Motor Accidents Insurance Commission; 2004.
-
- MAA. Guidelines for the management of whiplash associated disorders. Sydney: Motor accidents authority; 2007. p. 16.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources