The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by the initial tumor burden in the upper abdomen cephalad to the greater omentum
- PMID: 20022092
- PMCID: PMC4843127
- DOI: 10.1016/j.ygyno.2009.11.022
The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by the initial tumor burden in the upper abdomen cephalad to the greater omentum
Abstract
Objective: Our objective was to analyze the effect of surgical outcome on progression-free survival (PFS) and overall survival (OS) of patients with advanced ovarian carcinoma stratified by the initial presence and volume of upper abdominal disease cephalad to the greater omentum (UAD) found at the time of exploration.
Methods: We evaluated all patients with FIGO stage IIIC ovarian carcinoma who underwent primary cytoreduction followed by platinum-based chemotherapy at our institution between January 1989 and December 2006. The effect of surgical outcome was investigated using a time-to-event analysis. A Cox proportional hazards model was fit using clinical, surgical, and postoperative variables.
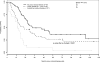
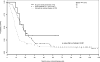
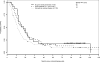
Results: We identified 526 evaluable patients. Optimal versus suboptimal cytoreduction was significantly associated with improved median PFS and OS in patients with no, minimal (<or=1 cm), and bulky (>1 cm) UAD. On multivariate analysis, patients with bulky UAD who underwent optimal cytoreduction had a 28% decreased risk of relapse (hazard ratio, 0.72; 95% confidence interval: 0.53-0.99; P=0.04) and a 33% decreased risk of death (hazard ratio, 0.67; 95% confidence interval: 0.47-0.96; P=0.03) compared to patients who underwent suboptimal cytoreduction.
Conclusion: The presence of large-volume disease found during surgical exploration does not preclude the benefit of optimal cytoreduction. The findings support the management strategy of maximizing surgical efforts with increasing tumor burden in patients with stage IIIC ovarian cancer. Prospective studies are needed to more precisely quantify tumor burden and accurately determine the specific impact of cytoreduction on outcome.
Conflict of interest statement
Oliver Zivanovic, MD: no conflicts of interest to declare
Camelia Sima: no conflicts of interest to declare
Alexia Iasonos, PhD: no conflicts of interest to declare
William J. Hoskins, MD: no conflicts of interest to declare
Pavani R. Pingle: no conflicts of interest to declare
Mario MM Leitao Jr, MD: Genzyme – consultant/speaker; Intuitive Surgical – surgical proctor
Yukio Sonoda, MD: Plasma Surgical – research support; Covidien – consultant; Genzyme – speaker
Nadeem R. Abu-Rustum, MD: no conflicts of interest to declare
Richard R. Barakat, MD: no conflicts of interest to declare
Dennis S. Chi, MD: Genzyme – speaker
Figures

References
-
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Gynecologic Oncology Group: Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. - PubMed
-
- Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. - PubMed
-
- Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–5. - PubMed
-
- Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Southwest Oncology Group; Gynecologic Oncology Group: Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21:2460–5. - PubMed
-
- Rose PG, Nerenstone S, Brady MF, Clarke-Pearson D, Olt G, Rubin SC, et al. Gynecologic Oncology Group: Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351:2489–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

