Insights into substrate binding at FeMo-cofactor in nitrogenase from the structure of an alpha-70(Ile) MoFe protein variant
- PMID: 20022118
- PMCID: PMC9186003
- DOI: 10.1016/j.jinorgbio.2009.11.009
Insights into substrate binding at FeMo-cofactor in nitrogenase from the structure of an alpha-70(Ile) MoFe protein variant
Abstract
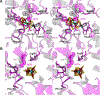
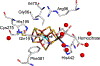
The X-ray crystal structure is presented for a nitrogenase MoFe protein where the alpha subunit residue at position 70 (alpha-70(Val)) has been substituted by the amino acid isoleucine (alpha-70(Ile)). Substitution of alpha-70(Val) by alpha-70(Ile) results in a MoFe protein that is hampered in its ability to reduce a range of substrates including acetylene and N(2), yet retains normal proton reduction activity. The 2.3A structure of the alpha-70(Ile) MoFe protein is compared to the alpha-70(Val) wild-type MoFe protein, revealing that the delta methyl group of alpha-70(Val) is positioned over Fe6 within the active site FeMo-cofactor. This work provides strong crystallographic support for the previously proposed model that substrates bind and are reduced at a single 4Fe-4S face of the FeMo-cofactor and that when alpha-70(Val) is substituted by alpha-70(Ile) access of substrates to Fe6 of this face is effectively blocked. Furthermore the detailed examination of the structure provides the basis for understanding the ability to trap and characterize hydrides in the variant, contributing significantly to our understanding of substrate access and substrate reduction at the FeMo-cofactor active site of nitrogenase.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
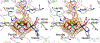
Similar articles
-
Alkyne substrate interaction within the nitrogenase MoFe protein.J Inorg Biochem. 2007 Nov;101(11-12):1642-8. doi: 10.1016/j.jinorgbio.2007.05.007. Epub 2007 May 29. J Inorg Biochem. 2007. PMID: 17610955 Free PMC article.
-
Interaction of acetylene and cyanide with the resting state of nitrogenase alpha-96-substituted MoFe proteins.Biochemistry. 2001 Nov 20;40(46):13816-25. doi: 10.1021/bi011571m. Biochemistry. 2001. PMID: 11705370
-
Role of the MoFe protein alpha-subunit histidine-195 residue in FeMo-cofactor binding and nitrogenase catalysis.Biochemistry. 1995 Mar 7;34(9):2798-808. doi: 10.1021/bi00009a008. Biochemistry. 1995. PMID: 7893691
-
Nitrogenase structure and function: a biochemical-genetic perspective.Annu Rev Microbiol. 1995;49:335-66. doi: 10.1146/annurev.mi.49.100195.002003. Annu Rev Microbiol. 1995. PMID: 8561464 Review.
-
Mechanism of Mo-dependent nitrogenase.Annu Rev Biochem. 2009;78:701-22. doi: 10.1146/annurev.biochem.78.070907.103812. Annu Rev Biochem. 2009. PMID: 19489731 Free PMC article. Review.
Cited by
-
Molybdenum imidazole citrate and bipyridine homocitrate in different oxidation states - balance between coordinated α-hydroxy and α-alkoxy groups.RSC Adv. 2019 Jan 2;9(1):519-528. doi: 10.1039/c8ra09134j. eCollection 2018 Dec 19. RSC Adv. 2019. PMID: 35521591 Free PMC article.
-
Structural characterization of the P1+ intermediate state of the P-cluster of nitrogenase.J Biol Chem. 2018 Jun 22;293(25):9629-9635. doi: 10.1074/jbc.RA118.002435. Epub 2018 May 2. J Biol Chem. 2018. PMID: 29720402 Free PMC article.
-
Reduction of N2 by supported tungsten clusters gives a model of the process by nitrogenase.Sci Rep. 2012;2:407. doi: 10.1038/srep00407. Epub 2012 May 14. Sci Rep. 2012. PMID: 22586517 Free PMC article.
-
A molecular pathway for the egress of ammonia produced by nitrogenase.Sci Rep. 2013 Nov 18;3:3237. doi: 10.1038/srep03237. Sci Rep. 2013. PMID: 24241241 Free PMC article.
-
Steric control of the Hi-CO MoFe nitrogenase complex revealed by stopped-flow infrared spectroscopy.Angew Chem Int Ed Engl. 2011 Jan 3;50(1):272-5. doi: 10.1002/anie.201005145. Angew Chem Int Ed Engl. 2011. PMID: 21120978 Free PMC article. No abstract available.
References
-
- Peters JW, Szilagyi RK, Curr. Opin. Chem. Biol 10 (2006) 101–108. - PubMed
-
- Barney BM, Lee HI, Dos Santos PC, Hoffman BM, Dean DR, Seefeldt LC, Dalton Trans (2006) 2277–2284. - PubMed
-
- Lee HI, Igarashi RY, Laryukhin M, Doan PE, Dos Santos PC, Dean DR, Seefeldt LC, Hoffman BM, J. Am. Chem. Soc 126 (2004) 9563–9569. - PubMed
-
- Dance J Am. Chem. Soc 126 (2004) 11852–11863. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

