The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition
- PMID: 20022176
- PMCID: PMC2814908
- DOI: 10.1016/j.pain.2009.11.017
The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition
Abstract
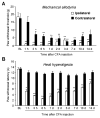
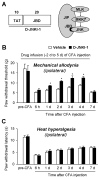
Peripheral inflammation induces persistent central sensitization characterized by mechanical allodynia and heat hyperalgesia that are mediated by distinct mechanisms. Compared to well-demonstrated mechanisms of heat hyperalgesia, mechanisms underlying the development of mechanical allodynia and contralateral pain are incompletely known. In this study, we investigated the distinct role of spinal JNK in heat hyperalgesia, mechanical allodynia, and contralateral pain in an inflammatory pain model. Intraplantar injection of complete Freund's adjuvant (CFA) induced bilateral mechanical allodynia but unilateral heat hyperalgesia. CFA also induced a bilateral activation (phosphorylation) of JNK in the spinal cord, and the phospho JNK1 (pJNK1) levels were much higher than that of pJNK2. Notably, both pJNK and JNK1 were expressed in GFAP-positive astrocytes. Intrathecal infusion of a selective peptide inhibitor of JNK, D-JNKI-1, starting before inflammation via an osmotic pump, reduced CFA-induced mechanical allodynia in the maintenance phase but had no effect on CFA-induced heat hyperalgesia. A bolus intrathecal injection of D-JNKI-1 or SP600126, a small molecule inhibitor of JNK also reversed mechanical allodynia bilaterally. In contrast, peripheral (intraplantar) administration of D-JNKI-1 reduced the induction of CFA-induced heat hyperalgesia but did not change mechanical allodynia. Finally, CFA-induced bilateral mechanical allodynia was attenuated in mice lacking JNK1 but not JNK2. Taken together, our data suggest that spinal JNK, in particular JNK1 plays an important role in the maintenance of persistent inflammatory pain. Our findings also reveal a unique role of JNK1 and astrocyte network in regulating tactile allodynia and contralateral pain.
Figures




Similar articles
-
Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia.J Neurochem. 2010 Oct;115(2):505-14. doi: 10.1111/j.1471-4159.2010.06946.x. Epub 2010 Aug 31. J Neurochem. 2010. PMID: 20722971 Free PMC article.
-
N-Methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain.Pain. 2010 Feb;148(2):237-246. doi: 10.1016/j.pain.2009.11.003. Epub 2009 Dec 11. Pain. 2010. PMID: 20005044 Free PMC article.
-
Electroacupuncture attenuates mechanical allodynia by suppressing the spinal JNK1/2 pathway in a rat model of inflammatory pain.Brain Res Bull. 2014 Sep;108:27-36. doi: 10.1016/j.brainresbull.2014.06.004. Epub 2014 Jul 7. Brain Res Bull. 2014. PMID: 25010483
-
Activation of JNK pathway in persistent pain.Neurosci Lett. 2008 Jun 6;437(3):180-3. doi: 10.1016/j.neulet.2008.03.017. Epub 2008 Mar 13. Neurosci Lett. 2008. PMID: 18455869 Free PMC article. Review.
-
Models and mechanisms of hyperalgesia and allodynia.Physiol Rev. 2009 Apr;89(2):707-58. doi: 10.1152/physrev.00025.2008. Physiol Rev. 2009. PMID: 19342617 Review.
Cited by
-
Emerging role of Toll-like receptors in the control of pain and itch.Neurosci Bull. 2012 Apr;28(2):131-44. doi: 10.1007/s12264-012-1219-5. Neurosci Bull. 2012. PMID: 22466124 Free PMC article. Review.
-
Protein kinase B/Akt is required for complete Freund's adjuvant-induced upregulation of Nav1.7 and Nav1.8 in primary sensory neurons.J Pain. 2013 Jun;14(6):638-47. doi: 10.1016/j.jpain.2013.01.778. Epub 2013 Apr 30. J Pain. 2013. PMID: 23642408 Free PMC article.
-
Tissue plasminogen activator contributes to morphine tolerance and induces mechanical allodynia via astrocytic IL-1β and ERK signaling in the spinal cord of mice.Neuroscience. 2013 Sep 5;247:376-85. doi: 10.1016/j.neuroscience.2013.05.018. Epub 2013 May 21. Neuroscience. 2013. PMID: 23707980 Free PMC article.
-
Analgesic effects of the cathepsin K inhibitor L-006235 in the monosodium iodoacetate model of osteoarthritis pain.Pain Rep. 2018 Oct 5;3(6):e685. doi: 10.1097/PR9.0000000000000685. eCollection 2018 Nov. Pain Rep. 2018. PMID: 30706033 Free PMC article.
-
Spinal Cord Glycine Transporter 2 Mediates Bilateral ST35 Acupoints Sensitization in Rats with Knee Osteoarthritis.Evid Based Complement Alternat Med. 2019 Feb 7;2019:7493286. doi: 10.1155/2019/7493286. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 30881475 Free PMC article.
References
-
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. - PubMed
-
- Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G. ‘Mirror pain’ in the formalin test: behavioral and 2-deoxyglucose studies. Pain. 1993;55:267–73. - PubMed
-
- Ambalavanar R, Dessem D, Moutanni A, Yallampalli C, Yallampalli U, Gangula P, Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neurosci. 2006;143:875–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

