Discovery of the mitotic selective chromatid segregation phenomenon and its implications for vertebrate development
- PMID: 20022232
- PMCID: PMC7241865
- DOI: 10.1016/j.ceb.2009.11.006
Discovery of the mitotic selective chromatid segregation phenomenon and its implications for vertebrate development
Abstract
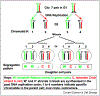
The asymmetric cell division process is required for cellular differentiation and embryonic development. Recent evidence obtained in Drosophila and C. elegans suggest that this process occurs by non-equivalent distribution of proteins or mRNA (intrinsic factors) to daughter cells, or by their differential exposure to cell extrinsic factors. In contrast, haploid fission yeast sister cells developmentally differ by inheriting sister chromatids that are differentiated by epigenetic means. Specifically, the act of DNA replication at the mating-type locus in yeast switches it's alternate alleles only in one specific member of chromosome 2 sister chromatids in nearly every chromosome replication cycle. To employ this kind of mechanism for cellular differentiation, strictly based on Watson-Crick structure of DNA in diploid organism, selective segregation mechanism is required to coordinate distribution of potentially differentiated sister chromatids to daughter cells. Genetic evidence to this postulate was fortuitously provided by the analysis of mitotic recombinants of chromosome 7 in mouse cells. Remarkably, the biased segregation occurs in some cell types but not in others and the process seems to be chromosome-specific. This review summarizes the discovery of selective chromatid segregation phenomenon and it suggests that such a process of Somatic Sister chromatid Imprinting and Selective chromatid Segregation (SSIS model) might explain development in eukaryotes, such as that of the body axis left-right visceral organs laterality specification in mice.
Published by Elsevier Ltd.
Figures

References
-
- Ephrussi A, Johnston D: Seeing is believing: the bicoid morphogen gradient matures. Cell 2004, 116:143–152. - PubMed
-
- Tabin C, Wolpert L: Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev 2007, 21:1433–1442. - PubMed
-
- Tickle C: Morphogen gradients in vertebrate limb development. Semin Cell Dev Biol 1999, 10:345–351. - PubMed
-
- Lin H: The stem-cell niche theory: lessons from flies. Nat Rev Genet 2002, 3:931–940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

