Hijacking HES1: how tumors co-opt the anti-differentiation strategies of quiescent cells
- PMID: 20022559
- PMCID: PMC2864914
- DOI: 10.1016/j.molmed.2009.11.001
Hijacking HES1: how tumors co-opt the anti-differentiation strategies of quiescent cells
Abstract
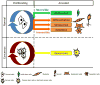
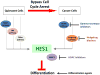
Quiescent and tumor cells share the ability to evade irreversible cell fates. Recent studies have shown that the transcriptional regulator Hairy and Enhancer of Split 1 (HES1) protects quiescent fibroblasts from differentiation or senescence. HES1 is highly expressed in rhabdomyosarcomas, and the inhibition of HES1 restores differentiation in these cells. Pathways that lead to elevated HES1 levels, such as the Notch and Hedgehog pathways, are frequently upregulated in tumors. Compounds that inhibit these pathways induce differentiation and apoptosis in cancer cells and several are in clinical trials. HES1 might repress gene expression in part by recruiting histone deacetylases (HDACs). HDACs inhibit differentiation, whereas histone deacetylase inhibitors (HDACis) induce differentiation or apoptosis in tumors and are also showing promise as therapeutics. Small molecules that directly target HES1 itself were recently identified. Here, we discuss the importance of HES1 function in quiescent and tumor cells. Elucidating the pathways that control quiescence could provide valuable information not only for treating cancer but also other diseases.
Figures
References
-
- Hanahan D, et al. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Holyoake T, et al. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. - PubMed
-
- Jorgensen HG, et al. Lonafarnib reduces the resistance of primitive quiescent CML cells to imatinib mesylate in vitro. Leukemia. 2005;19:1184–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

