TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis
- PMID: 20022921
- PMCID: PMC2814103
- DOI: 10.1093/jxb/erp331
TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis
Abstract
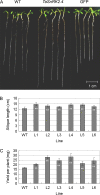
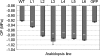
Osmotic stresses such as drought, salinity, and cold are major environmental factors that limit agricultural productivity worldwide. Protein phosphorylation/dephosphorylation are major signalling events induced by osmotic stress in higher plants. Sucrose non-fermenting 1-related protein kinase2 family members play essential roles in response to hyperosmotic stresses in Arabidopsis, rice, and maize. In this study, the function of TaSnRK2.4 in drought, salt, and freezing stresses in Arabidopsis was characterized. A translational fusion protein of TaSnRK2.4 with green fluorescent protein showed subcellular localization in the cell membrane, cytoplasm, and nucleus. To examine the role of TaSnRK2.4 under various environmental stresses, transgenic Arabidopsis plants overexpressing wheat TaSnRK2.4 under control of the cauliflower mosaic virus 35S promoter were generated. Overexpression of TaSnRK2.4 resulted in delayed seedling establishment, longer primary roots, and higher yield under normal growing conditions. Transgenic Arabidopsis overexpressing TaSnRK2.4 had enhanced tolerance to drought, salt, and freezing stresses, which were simultaneously supported by physiological results, including decreased rate of water loss, enhanced higher relative water content, strengthened cell membrane stability, improved photosynthesis potential, and significantly increased osmotic potential. The results show that TaSnRK2.4 is involved in the regulation of enhanced osmotic potential, growth, and development under both normal and stress conditions, and imply that TaSnRK2.4 is a multifunctional regulatory factor in Arabidopsis. Since the overexpression of TaSnRK2.4 can significantly strengthen tolerance to drought, salt, and freezing stresses and does not retard the growth of transgenic Arabidopsis plants under well-watered conditions, TaSnRK2.4 could be utilized in transgenic breeding to improve abiotic stresses in crops.
Figures








References
-
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Critical Reviews in Plant Science. 2005;24:23–58.
-
- Boudsocq M, Barbier-Brygoo H, Lauriere C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. Journal of Biolical Chemistry. 2004;279:41758–41766. - PubMed
-
- Boudsocq M, Droillard MJ, Barbier-Brygoo H, Lauriere C. Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Molecular Biology. 2007;63:491–503. - PubMed
-
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. - PubMed
-
- Bray E. Plant responses to water deficit. Trends in Plant Science. 1997;2:48–54.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

