Minor modifications of the C-terminal helix reschedule the favored chemical reactions catalyzed by theta class glutathione transferase T1-1
- PMID: 20022951
- PMCID: PMC2820791
- DOI: 10.1074/jbc.M109.074757
Minor modifications of the C-terminal helix reschedule the favored chemical reactions catalyzed by theta class glutathione transferase T1-1
Abstract
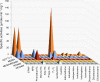
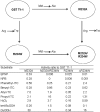
Adaptive responses to novel toxic challenges provide selective advantages to organisms in evolution. Glutathione transferases (GSTs) play a pivotal role in the cellular defense because they are main contributors to the inactivation of genotoxic compounds of exogenous as well as of endogenous origins. GSTs are promiscuous enzymes catalyzing a variety of chemical reactions with numerous alternative substrates. Despite broad substrate acceptance, individual GSTs display pronounced selectivities such that only a limited number of substrates are transformed with high catalytic efficiency. The present study shows that minor structural changes in the C-terminal helix of mouse GST T1-1 induce major changes in the substrate-activity profile of the enzyme to favor novel chemical reactions and to suppress other reactions catalyzed by the parental enzyme.
Figures




Similar articles
-
An ensemble of theta class glutathione transferases with novel catalytic properties generated by stochastic recombination of fragments of two mammalian enzymes.J Mol Biol. 2002 Apr 19;318(1):59-70. doi: 10.1016/S0022-2836(02)00032-3. J Mol Biol. 2002. PMID: 12054768
-
Structural determinants of glutathione transferases with azathioprine activity identified by DNA shuffling of alpha class members.J Mol Biol. 2008 Feb 1;375(5):1365-79. doi: 10.1016/j.jmb.2007.11.034. Epub 2007 Nov 19. J Mol Biol. 2008. PMID: 18155239
-
Residue 234 is a master switch of the alternative-substrate activity profile of human and rodent theta class glutathione transferase T1-1.Biochim Biophys Acta. 2010 Apr;1800(4):466-73. doi: 10.1016/j.bbagen.2010.01.003. Epub 2010 Jan 25. Biochim Biophys Acta. 2010. PMID: 20097269
-
Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily.Biochem J. 2001 Nov 15;360(Pt 1):1-16. doi: 10.1042/0264-6021:3600001. Biochem J. 2001. PMID: 11695986 Free PMC article. Review.
-
Mammalian class theta GST and differential susceptibility to carcinogens: a review.Mutat Res. 2000 Oct;463(3):247-83. doi: 10.1016/s1383-5742(00)00050-8. Mutat Res. 2000. PMID: 11018744 Review.
Cited by
-
Metabolism of isothiocyanates in individuals with positive and null GSTT1 and M1 genotypes after drinking watercress juice.Clin Nutr. 2010 Dec;29(6):813-8. doi: 10.1016/j.clnu.2010.06.010. Epub 2010 Jul 24. Clin Nutr. 2010. PMID: 20656381 Free PMC article.
-
Genetic variations in human glutathione transferase enzymes: significance for pharmacology and toxicology.Hum Genomics Proteomics. 2010 Jun 13;2010:876940. doi: 10.4061/2010/876940. Hum Genomics Proteomics. 2010. PMID: 20981235 Free PMC article.
-
Effects of Substrate-Binding Site Residues on the Biochemical Properties of a Tau Class Glutathione S-Transferase from Oryza sativa.Genes (Basel). 2019 Dec 24;11(1):25. doi: 10.3390/genes11010025. Genes (Basel). 2019. PMID: 31878175 Free PMC article.
-
Glutathione-binding site of a bombyx mori theta-class glutathione transferase.PLoS One. 2014 May 21;9(5):e97740. doi: 10.1371/journal.pone.0097740. eCollection 2014. PLoS One. 2014. PMID: 24848539 Free PMC article.
-
Molecular evolution and the role of oxidative stress in the expansion and functional diversification of cytosolic glutathione transferases.BMC Evol Biol. 2010 Sep 15;10:281. doi: 10.1186/1471-2148-10-281. BMC Evol Biol. 2010. PMID: 20843339 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

