Minor modifications of the C-terminal helix reschedule the favored chemical reactions catalyzed by theta class glutathione transferase T1-1
- PMID: 20022951
- PMCID: PMC2820791
- DOI: 10.1074/jbc.M109.074757
Minor modifications of the C-terminal helix reschedule the favored chemical reactions catalyzed by theta class glutathione transferase T1-1
Abstract
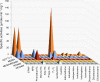
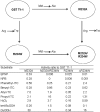
Adaptive responses to novel toxic challenges provide selective advantages to organisms in evolution. Glutathione transferases (GSTs) play a pivotal role in the cellular defense because they are main contributors to the inactivation of genotoxic compounds of exogenous as well as of endogenous origins. GSTs are promiscuous enzymes catalyzing a variety of chemical reactions with numerous alternative substrates. Despite broad substrate acceptance, individual GSTs display pronounced selectivities such that only a limited number of substrates are transformed with high catalytic efficiency. The present study shows that minor structural changes in the C-terminal helix of mouse GST T1-1 induce major changes in the substrate-activity profile of the enzyme to favor novel chemical reactions and to suppress other reactions catalyzed by the parental enzyme.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

