Deficiency in APOBEC2 leads to a shift in muscle fiber type, diminished body mass, and myopathy
- PMID: 20022958
- PMCID: PMC2844160
- DOI: 10.1074/jbc.M109.052977
Deficiency in APOBEC2 leads to a shift in muscle fiber type, diminished body mass, and myopathy
Abstract
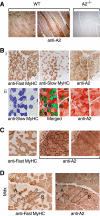
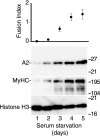
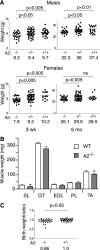
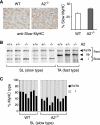
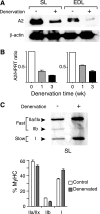
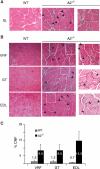
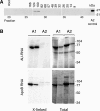
The apoB RNA-editing enzyme, catalytic polypeptide-like (APOBEC) family of proteins includes APOBEC1, APOBEC3, and activation-induced deaminase, all of which are zinc-dependent cytidine deaminases active on polynucleotides and involved in RNA editing or DNA mutation. In contrast, the biochemical and physiological functions of APOBEC2, a muscle-specific member of the family, are unknown, although it has been speculated, like APOBEC1, to be an RNA-editing enzyme. Here, we show that, although expressed widely in striated muscle (with levels peaking late during myoblast differentiation), APOBEC2 is preferentially associated with slow-twitch muscle, with its abundance being considerably greater in soleus compared with gastrocnemius muscle and, within soleus muscle, in slow as opposed to fast muscle fibers. Its abundance also decreases following muscle denervation. We further show that APOBEC2-deficient mice harbor a markedly increased ratio of slow to fast fibers in soleus muscle and exhibit an approximately 15-20% reduction in body mass from birth onwards, with elderly mutant animals revealing clear histological evidence of a mild myopathy. Thus, APOBEC2 is essential for normal muscle development and maintenance of fiber-type ratios; although its molecular function remains to be identified, biochemical analyses do not especially argue for any role in RNA editing.
Figures

Similar articles
-
Apobec2 deficiency causes mitochondrial defects and mitophagy in skeletal muscle.FASEB J. 2018 Mar;32(3):1428-1439. doi: 10.1096/fj.201700493R. Epub 2018 Jan 3. FASEB J. 2018. PMID: 29127187 Free PMC article.
-
APOBEC2 safeguards skeletal muscle cell fate through binding chromatin and regulating transcription of non-muscle genes during myoblast differentiation.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2312330121. doi: 10.1073/pnas.2312330121. Epub 2024 Apr 16. Proc Natl Acad Sci U S A. 2024. PMID: 38625936 Free PMC article.
-
APOBEC2 negatively regulates myoblast differentiation in muscle regeneration.Int J Biochem Cell Biol. 2017 Apr;85:91-101. doi: 10.1016/j.biocel.2017.02.005. Epub 2017 Feb 12. Int J Biochem Cell Biol. 2017. PMID: 28215905
-
Mice deficient in APOBEC2 and APOBEC3.Mol Cell Biol. 2005 Aug;25(16):7270-7. doi: 10.1128/MCB.25.16.7270-7277.2005. Mol Cell Biol. 2005. PMID: 16055735 Free PMC article.
-
The role of hedgehog proteins in vertebrate slow and fast skeletal muscle patterning.Acta Physiol Scand. 1998 Jul;163(3):S7-10. doi: 10.1046/j.1365-201X.1998.1630s30S7.x. Acta Physiol Scand. 1998. PMID: 9715744 Review. No abstract available.
Cited by
-
APOBEC4 Enhances the Replication of HIV-1.PLoS One. 2016 Jun 1;11(6):e0155422. doi: 10.1371/journal.pone.0155422. eCollection 2016. PLoS One. 2016. PMID: 27249646 Free PMC article.
-
Non-Coding RNA Editing in Cancer Pathogenesis.Cancers (Basel). 2020 Jul 8;12(7):1845. doi: 10.3390/cancers12071845. Cancers (Basel). 2020. PMID: 32650588 Free PMC article. Review.
-
Evolutionary Comparative Analyses of DNA-Editing Enzymes of the Immune System: From 5-Dimensional Description of Protein Structures to Immunological Insights and Applications to Protein Engineering.Front Immunol. 2021 May 31;12:642343. doi: 10.3389/fimmu.2021.642343. eCollection 2021. Front Immunol. 2021. PMID: 34135887 Free PMC article.
-
Mutator effects and mutation signatures of editing deaminases produced in bacteria and yeast.Biochemistry (Mosc). 2011 Jan;76(1):131-46. doi: 10.1134/s0006297911010135. Biochemistry (Mosc). 2011. PMID: 21568845 Free PMC article.
-
PAX7 Balances the Cell Cycle Progression via Regulating Expression of Dnmt3b and Apobec2 in Differentiating PSCs.Cells. 2021 Aug 26;10(9):2205. doi: 10.3390/cells10092205. Cells. 2021. PMID: 34571854 Free PMC article.
References
-
- Conticello S. G., Thomas C. J., Petersen-Mahrt S. K., Neuberger M. S. (2005) Mol. Biol. Evol. 22, 367–377 - PubMed
-
- Navaratnam N., Sarwar R. (2006) Int. J. Hematol. 83, 195–200 - PubMed
-
- Liao W., Hong S. H., Chan B. H., Rudolph F. B., Clark S. C., Chan L. (1999) Biochem. Biophys. Res. Commun. 260, 398–404 - PubMed
-
- Anant S., Mukhopadhyay D., Sankaranand V., Kennedy S., Henderson J. O., Davidson N. O. (2001) Am. J. Physiol. Cell Physiol. 281, C1904–C1916 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

