Heparan sulfate is required for embryonic stem cells to exit from self-renewal
- PMID: 20022960
- PMCID: PMC2820816
- DOI: 10.1074/jbc.M109.066837
Heparan sulfate is required for embryonic stem cells to exit from self-renewal
Erratum in
-
Heparan sulfate is required for embryonic stem cells to exit from self-renewal.J Biol Chem. 2015 Sep 18;290(38):23023. doi: 10.1074/jbc.A109.066837. J Biol Chem. 2015. PMID: 26386045 Free PMC article. No abstract available.
Abstract
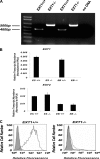
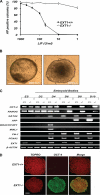
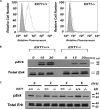
Pluripotent embryonic stem cells (ESCs) must select between alternative fates of self-renewal and lineage commitment at each division during continuous proliferation. Heparan sulfate (HS) is a highly sulfated polysaccharide and is present abundantly on the ESC surface. In this study, we investigated the role of HS in ESC self-renewal by examining Ext1(-/-) ESCs that are deficient in HS. We found that Ext1(-/-) ESCs retained their self-renewal potential but failed to transit from self-renewal to differentiation upon removal of leukemia inhibitory factor. Furthermore, we found that the aberrant cell fate commitment is caused by defects in fibroblast growth factor signaling, which directly retained high expression of the pluripotency gene Nanog in Ext1(-/-) ESCs. Therefore, our studies identified and defined HS as a novel factor that controls ESC fate commitment and also delineates that HS facilitates fibroblast growth factor signaling, which, in turn, inhibits Nanog expression and commits ESCs to lineage differentiation.
Figures



Similar articles
-
Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells.J Biol Chem. 2012 Jun 29;287(27):22691-700. doi: 10.1074/jbc.M112.368241. Epub 2012 May 3. J Biol Chem. 2012. PMID: 22556407 Free PMC article.
-
Heparan sulfation-dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state.Stem Cells. 2010 Feb;28(2):191-200. doi: 10.1002/stem.265. Stem Cells. 2010. PMID: 19937756
-
Heparan sulfate: a key regulator of embryonic stem cell fate.Biol Chem. 2013 Jun;394(6):741-51. doi: 10.1515/hsz-2012-0353. Biol Chem. 2013. PMID: 23370908 Free PMC article. Review.
-
Influencing hematopoietic differentiation of mouse embryonic stem cells using soluble heparin and heparan sulfate saccharides.J Biol Chem. 2011 Feb 25;286(8):6241-52. doi: 10.1074/jbc.M110.178483. Epub 2010 Dec 10. J Biol Chem. 2011. PMID: 21148566 Free PMC article.
-
Heparan sulfate biosynthesis enzymes in embryonic stem cell biology.J Histochem Cytochem. 2012 Dec;60(12):943-9. doi: 10.1369/0022155412465090. Epub 2012 Oct 4. J Histochem Cytochem. 2012. PMID: 23042480 Free PMC article. Review.
Cited by
-
Long-lived engineering of glycans to direct stem cell fate.Angew Chem Int Ed Engl. 2015 Jan 26;54(5):1466-70. doi: 10.1002/anie.201409258. Epub 2014 Dec 4. Angew Chem Int Ed Engl. 2015. PMID: 25476911 Free PMC article.
-
Differential plasticity of epiblast and primitive endoderm precursors within the ICM of the early mouse embryo.Development. 2012 Jan;139(1):129-39. doi: 10.1242/dev.067702. Epub 2011 Nov 17. Development. 2012. PMID: 22096072 Free PMC article.
-
Undersulfation of heparan sulfate restricts differentiation potential of mouse embryonic stem cells.J Biol Chem. 2012 Mar 30;287(14):10853-62. doi: 10.1074/jbc.M111.337030. Epub 2012 Feb 1. J Biol Chem. 2012. PMID: 22298785 Free PMC article.
-
Defining synthetic surfaces for human pluripotent stem cell culture.Cell Regen. 2013 Nov 22;2(1):7. doi: 10.1186/2045-9769-2-7. eCollection 2013. Cell Regen. 2013. PMID: 25408879 Free PMC article. Review.
-
Sulfation of Glycosaminoglycans Modulates the Cell Cycle of Embryonic Mouse Spinal Cord Neural Stem Cells.Front Cell Dev Biol. 2021 Jun 8;9:643060. doi: 10.3389/fcell.2021.643060. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34169071 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

